Input interpretation

highest element | by boiling point
Result

rhenium (5596 °C (degrees Celsius))
Periodic table location
Periodic table location
Image

Image
Basic elemental properties
![atomic symbol | Re atomic number | 75 short electronic configuration | [Xe]6s^24f^145d^5 Aufbau diagram | 5d 4f 6s block | d group | 7 period | 6 atomic mass | 186.207 u (unified atomic mass units)](../image_source/a95d7eb4ca7cfe815b3e4179cbda5ede.png)
atomic symbol | Re atomic number | 75 short electronic configuration | [Xe]6s^24f^145d^5 Aufbau diagram | 5d 4f 6s block | d group | 7 period | 6 atomic mass | 186.207 u (unified atomic mass units)
Thermodynamic properties
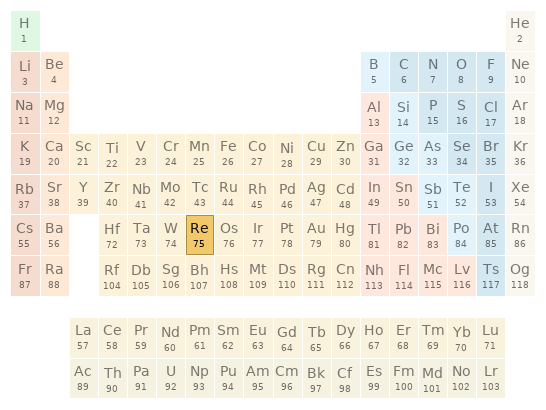
phase at STP | solid melting point | 3186 °C (rank: 3rd) boiling point | 5596 °C (rank: 1st) molar heat of fusion | 33 kJ/mol (rank: 7th) molar heat of vaporization | 705 kJ/mol (rank: 4th) specific heat at STP | 137 J/(kg K) (rank: 73rd) (properties at standard conditions)
Material properties
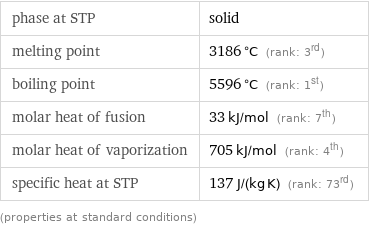
density | 21.02 g/cm^3 (rank: 4th) liquid density | 18.9 g/cm^3 molar volume | 8.8586 cm^3/mol Mohs hardness | 7 (≈ quartz) Vickers hardness | 2450 MPa (rank: 6th) Brinell hardness | 1320 MPa (rank: 11th) bulk modulus | 370 GPa (rank: 2nd) shear modulus | 178 GPa Young's modulus | 463 GPa (rank: 2nd) Poisson ratio | 0.3 (rank: 22nd) sound speed | 4700 m/s (rank: 17th) thermal expansion | 6.2×10^-6 K^(-1) (rank: 55th) thermal conductivity | 48 W/(m K) (rank: 36th) (properties at standard conditions)
Electromagnetic properties
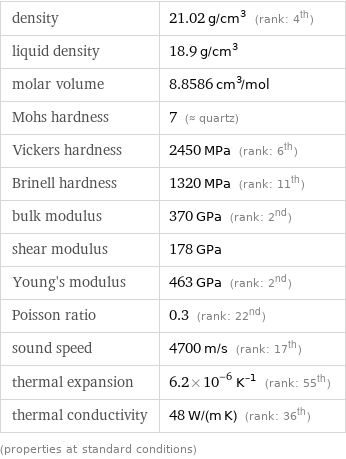
electrical type | conductor resistivity | 1.8×10^-7 Ω m (ohm meters) electrical conductivity | 5.6×10^6 S/m (siemens per meter) magnetic type | paramagnetic volume magnetic susceptibility | 9.59×10^-5 mass magnetic susceptibility | 4.56×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 8.49×10^-10 m^3/mol (cubic meters per mole) work function | 4.72 eV (Polycrystalline) threshold frequency | 1.141×10^15 Hz (hertz) superconducting point | 1.7 K (kelvins) color | (gray)
Reactivity
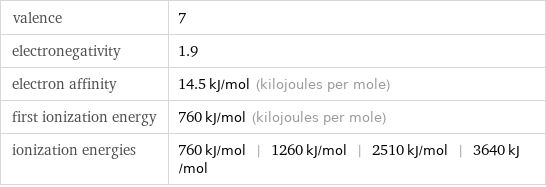
valence | 7 electronegativity | 1.9 electron affinity | 14.5 kJ/mol (kilojoules per mole) first ionization energy | 760 kJ/mol (kilojoules per mole) ionization energies | 760 kJ/mol | 1260 kJ/mol | 2510 kJ/mol | 3640 kJ/mol

Reactivity
Atomic properties

term symbol | ^6S_(5/2) atomic radius | 135 pm covalent radius | 151 pm (electronic ground state properties)
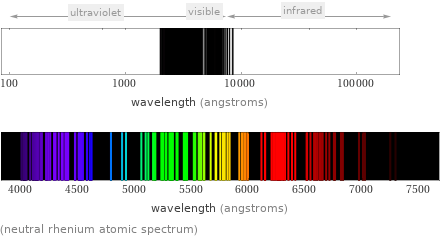
(neutral rhenium atomic spectrum)
Abundances

universe abundance | 2×10^-8 mass% (rank: 79th) solar abundance | 1×10^-8 mass% (rank: 71st) meteorite abundance | 4.9×10^-6 mass% (rank: 70th) crust abundance | 2.6×10^-7 mass% (rank: 76th) ocean abundance | 1×10^-10 mass% (rank: 60th) universe molar abundance | 1×10^-10 mol% (rank: 78th) solar molar abundance | 5×10^-11 mol% (rank: 75th) meteorite molar abundance | 5×10^-7 mol% (rank: 71st) ocean molar abundance | 3.3×10^-12 mol% (rank: 75th) crust molar abundance | 3×10^-8 mol% (rank: 76th)
Nuclear properties
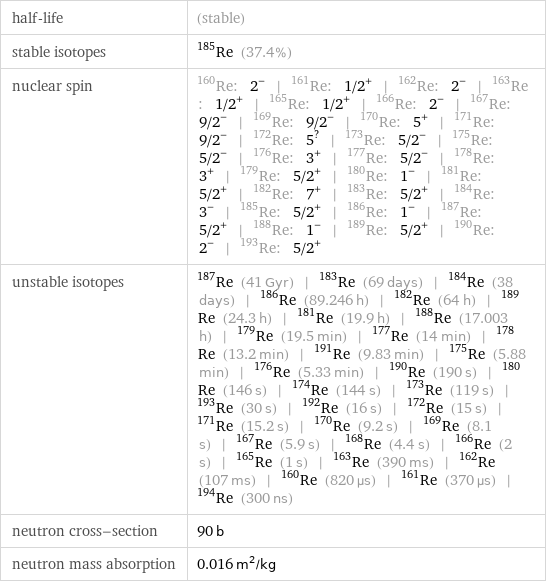
half-life | (stable) stable isotopes | Re-185 (37.4%) nuclear spin | Re-160: 2^- | Re-161: 1/2^+ | Re-162: 2^- | Re-163: 1/2^+ | Re-165: 1/2^+ | Re-166: 2^- | Re-167: 9/2^- | Re-169: 9/2^- | Re-170: 5^+ | Re-171: 9/2^- | Re-172: 5^? | Re-173: 5/2^- | Re-175: 5/2^- | Re-176: 3^+ | Re-177: 5/2^- | Re-178: 3^+ | Re-179: 5/2^+ | Re-180: 1^- | Re-181: 5/2^+ | Re-182: 7^+ | Re-183: 5/2^+ | Re-184: 3^- | Re-185: 5/2^+ | Re-186: 1^- | Re-187: 5/2^+ | Re-188: 1^- | Re-189: 5/2^+ | Re-190: 2^- | Re-193: 5/2^+ unstable isotopes | Re-187 (41 Gyr) | Re-183 (69 days) | Re-184 (38 days) | Re-186 (89.246 h) | Re-182 (64 h) | Re-189 (24.3 h) | Re-181 (19.9 h) | Re-188 (17.003 h) | Re-179 (19.5 min) | Re-177 (14 min) | Re-178 (13.2 min) | Re-191 (9.83 min) | Re-175 (5.88 min) | Re-176 (5.33 min) | Re-190 (190 s) | Re-180 (146 s) | Re-174 (144 s) | Re-173 (119 s) | Re-193 (30 s) | Re-192 (16 s) | Re-172 (15 s) | Re-171 (15.2 s) | Re-170 (9.2 s) | Re-169 (8.1 s) | Re-167 (5.9 s) | Re-168 (4.4 s) | Re-166 (2 s) | Re-165 (1 s) | Re-163 (390 ms) | Re-162 (107 ms) | Re-160 (820 µs) | Re-161 (370 µs) | Re-194 (300 ns) neutron cross-section | 90 b neutron mass absorption | 0.016 m^2/kg
Identifiers
CAS number | 7440-15-5 PubChem CID number | 23947
Elements ranked by boiling point

1 | rhenium | 5596 °C 2 | tungsten | 5555 °C 3 | tantalum | 5458 °C 4 | osmium | 5012 °C 5 | thorium | 4820 °C 6 | niobium | 4744 °C 7 | molybdenum | 4639 °C 8 | hafnium | 4603 °C 9 | iridium | 4428 °C 10 | zirconium | 4409 °C ⋮