Input interpretation
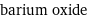
barium oxide
Chemical names and formulas
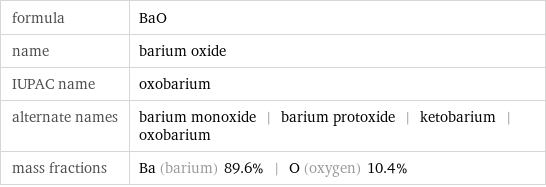
formula | BaO name | barium oxide IUPAC name | oxobarium alternate names | barium monoxide | barium protoxide | ketobarium | oxobarium mass fractions | Ba (barium) 89.6% | O (oxygen) 10.4%
Lewis structure

Draw the Lewis structure of barium oxide. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the barium (n_Ba, val = 2) and oxygen (n_O, val = 6) atoms: n_Ba, val + n_O, val = 8 Calculate the number of electrons needed to completely fill the valence shells for barium (n_Ba, full = 4) and oxygen (n_O, full = 8): n_Ba, full + n_O, full = 12 Subtracting these two numbers shows that 12 - 8 = 4 bonding electrons are needed. Each bond has two electrons, so in addition to the 1 bond already present in the diagram add 1 bond. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 1 bond by pairing electrons between adjacent highlighted atoms: Answer: | |
Basic properties
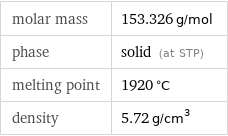
molar mass | 153.326 g/mol phase | solid (at STP) melting point | 1920 °C density | 5.72 g/cm^3
Units

Solid properties (at STP)
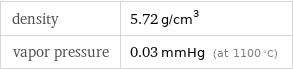
density | 5.72 g/cm^3 vapor pressure | 0.03 mmHg (at 1100 °C)
Units

Thermodynamic properties
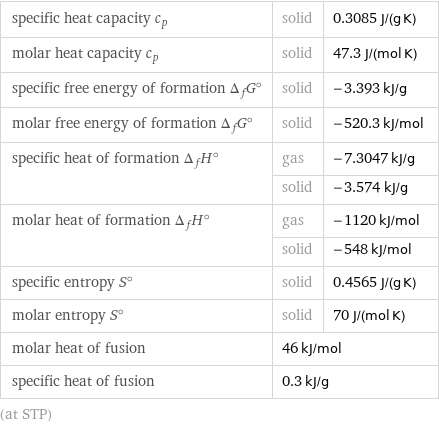
specific heat capacity c_p | solid | 0.3085 J/(g K) molar heat capacity c_p | solid | 47.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -3.393 kJ/g molar free energy of formation Δ_fG° | solid | -520.3 kJ/mol specific heat of formation Δ_fH° | gas | -7.3047 kJ/g | solid | -3.574 kJ/g molar heat of formation Δ_fH° | gas | -1120 kJ/mol | solid | -548 kJ/mol specific entropy S° | solid | 0.4565 J/(g K) molar entropy S° | solid | 70 J/(mol K) molar heat of fusion | 46 kJ/mol | specific heat of fusion | 0.3 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1304-28-5 PubChem CID number | 62392 PubChem SID number | 24853391 SMILES identifier | O=[Ba] InChI identifier | InChI=1/Ba.O/rBaO/c1-2 RTECS number | CQ9800000 MDL number | MFCD00003453](../image_source/e6853c8f500d90cb6aff9b063668bc4e.png)
CAS number | 1304-28-5 PubChem CID number | 62392 PubChem SID number | 24853391 SMILES identifier | O=[Ba] InChI identifier | InChI=1/Ba.O/rBaO/c1-2 RTECS number | CQ9800000 MDL number | MFCD00003453
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other