Input interpretation
ammonium tetrachloroaluminate | strontium phosphide
Chemical names and formulas
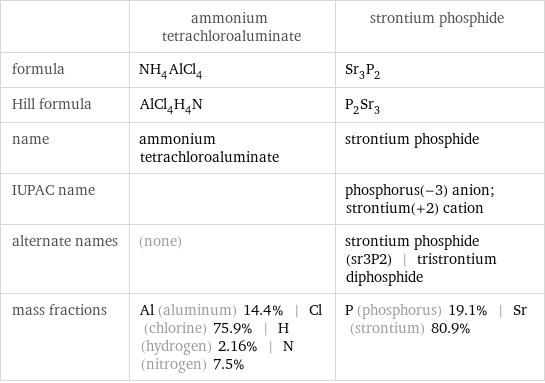
| ammonium tetrachloroaluminate | strontium phosphide formula | NH_4AlCl_4 | Sr_3P_2 Hill formula | AlCl_4H_4N | P_2Sr_3 name | ammonium tetrachloroaluminate | strontium phosphide IUPAC name | | phosphorus(-3) anion; strontium(+2) cation alternate names | (none) | strontium phosphide (sr3P2) | tristrontium diphosphide mass fractions | Al (aluminum) 14.4% | Cl (chlorine) 75.9% | H (hydrogen) 2.16% | N (nitrogen) 7.5% | P (phosphorus) 19.1% | Sr (strontium) 80.9%
Structure diagrams
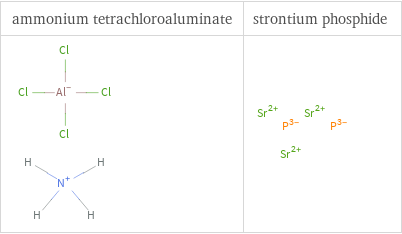
Structure diagrams
Basic properties

| ammonium tetrachloroaluminate | strontium phosphide molar mass | 186.8 g/mol | 324.8 g/mol melting point | 304 °C | density | | 2.68 g/cm^3
Units
