Input interpretation
lindane
Chemical names and formulas
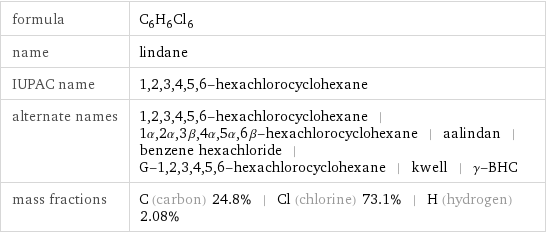
formula | C_6H_6Cl_6 name | lindane IUPAC name | 1, 2, 3, 4, 5, 6-hexachlorocyclohexane alternate names | 1, 2, 3, 4, 5, 6-hexachlorocyclohexane | 1α, 2α, 3β, 4α, 5α, 6β-hexachlorocyclohexane | aalindan | benzene hexachloride | G-1, 2, 3, 4, 5, 6-hexachlorocyclohexane | kwell | γ-BHC mass fractions | C (carbon) 24.8% | Cl (chlorine) 73.1% | H (hydrogen) 2.08%
Lewis structure
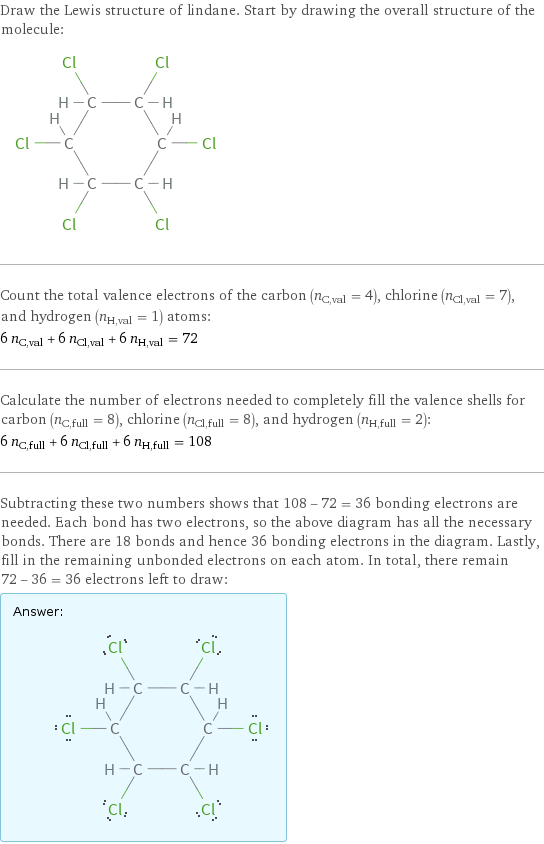
Draw the Lewis structure of lindane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), and hydrogen (n_H, val = 1) atoms: 6 n_C, val + 6 n_Cl, val + 6 n_H, val = 72 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), and hydrogen (n_H, full = 2): 6 n_C, full + 6 n_Cl, full + 6 n_H, full = 108 Subtracting these two numbers shows that 108 - 72 = 36 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 18 bonds and hence 36 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 72 - 36 = 36 electrons left to draw: Answer: | |
3D structure
3D structure
Basic properties
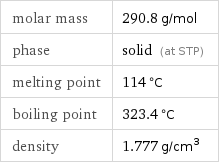
molar mass | 290.8 g/mol phase | solid (at STP) melting point | 114 °C boiling point | 323.4 °C density | 1.777 g/cm^3
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | 3.8 predicted LogP hydrophobicity | 3.94 predicted LogS | -4.73
Basic drug properties

approval status | approved | small molecule drug categories | antiscabies agent | insecticide | scabicide dosage forms | topical: emulsion | topical: lotion | topical: shampoo

brand names | aalindan | aficide | agrocide | agrocide III | agrocide WP | ameisenmittel merck | ameisentod | aparasin | aphtiria | aplidal | arbitex | atlas steward | BBH | ben-hex | bentox 10 | benzene hexachloride | bexol | celanex | chloresene | codechine | DBH | detmol-extrakt | devoran | dol granule | drilltox-spezial aglukon | entomoxan | esoderm | fumite lindane | gamacid | gamene | gamma-BHC dust | gamma-col | gamma-HCH dust | gammalin | gammalin 20 | gammasan | gammaterr | gammexane | gexane | HCCH | HCH | HGI | heclotox | hexa | hexachloran | hexachlorane | hexachlorocyclohexane | hexatox | hexaverm | hexicide | hexit | hexyclan | hortex | indane | isotox | jacutin | kokotine | kwell | lendine | lentox | lidenal | lindafor | lindatox | lindex | lindosep | lintox | linvur | lorexane | milbol 49 | mszycol | murfume grain store smoke | neo-scabicidol | new kotol | nexen FB | nexit | nexit-stark | nexol-E | nicochloran | omnitox | ovadziak | owadziak | PMS lindane | pedraczak | pflanzol | quellada | sang-«gamma» | scabene | scabene lotion | spritz-rapidin | spruehpflanzol | streunex | TAP 85 | thionex | tri-6 | viton | beta-BHC | beta-hexachlorocyclohexane | beta-lindane
Solid properties (at STP)
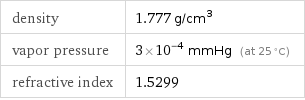
density | 1.777 g/cm^3 vapor pressure | 3×10^-4 mmHg (at 25 °C) refractive index | 1.5299
Units

Thermodynamic properties
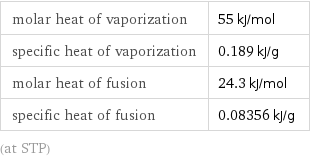
molar heat of vaporization | 55 kJ/mol specific heat of vaporization | 0.189 kJ/g molar heat of fusion | 24.3 kJ/mol specific heat of fusion | 0.08356 kJ/g (at STP)
Chemical identifiers
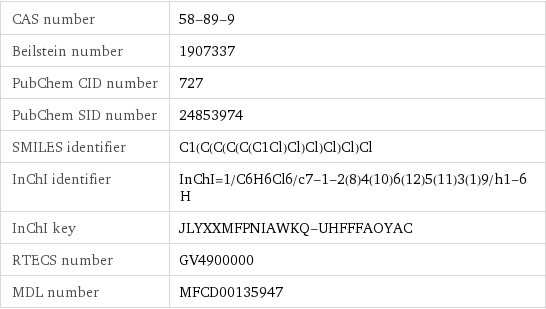
CAS number | 58-89-9 Beilstein number | 1907337 PubChem CID number | 727 PubChem SID number | 24853974 SMILES identifier | C1(C(C(C(C(C1Cl)Cl)Cl)Cl)Cl)Cl InChI identifier | InChI=1/C6H6Cl6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-6H InChI key | JLYXXMFPNIAWKQ-UHFFFAOYAC RTECS number | GV4900000 MDL number | MFCD00135947
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 1 NFPA reactivity rating | 0
Safety properties

flash point | 150 °C
DOT hazard class | 6.1 DOT numbers | 2761
Toxicity properties

lethal dosage | 76 mg/kg (oral dose for rats) short-term exposure limit | 1.5 mg/m^3

probable lethal dose for man | 30 mL (milliliters) long-term exposure limit | 0.5 mg/m^3 (over 8 hours) RTECS classes | agricultural chemical and pesticide | tumorigen | drug | mutagen | reproductive effector | human data