Input interpretation

rare earth metals | Néel point
Summary
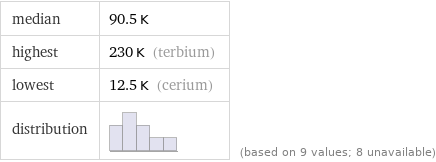
median | 90.5 K highest | 230 K (terbium) lowest | 12.5 K (cerium) distribution | | (based on 9 values; 8 unavailable)
Entities with missing values
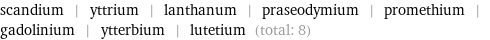
scandium | yttrium | lanthanum | praseodymium | promethium | gadolinium | ytterbium | lutetium (total: 8)
Distribution plots
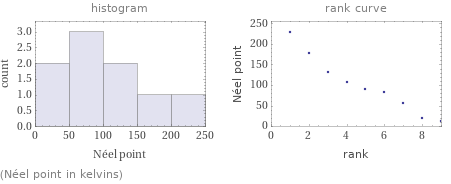
(Néel point in kelvins)
Néel point rankings
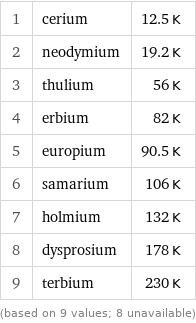
1 | cerium | 12.5 K 2 | neodymium | 19.2 K 3 | thulium | 56 K 4 | erbium | 82 K 5 | europium | 90.5 K 6 | samarium | 106 K 7 | holmium | 132 K 8 | dysprosium | 178 K 9 | terbium | 230 K (based on 9 values; 8 unavailable)
Unit conversions for median Néel point 90.5 K
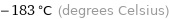
-183 °C (degrees Celsius)

-297 °F (degrees Fahrenheit)

163 °R (degrees Rankine)
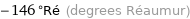
-146 °Ré (degrees Réaumur)

-88.4 °Rø (degrees Rømer)
Comparison for median Néel point 90.5 K

0.2 K above boiling point of oxygen (-182.9 °C)
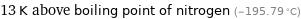
13 K above boiling point of nitrogen (-195.79 °C)
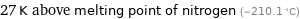
27 K above melting point of nitrogen (-210.1 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 7.8 meV (millielectronvolts)
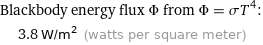
Blackbody energy flux Φ from Φ = σT^4: | 3.8 W/m^2 (watts per square meter)
Thermodynamic properties

phase at STP | all | solid melting point | median | 1313 °C | highest | 1663 °C (lutetium) | lowest | 798 °C (cerium) | distribution | boiling point | median | 3000 °C | highest | 3464 °C (lanthanum) | lowest | 1196 °C (ytterbium) | distribution | molar heat of fusion | median | 10 kJ/mol | highest | 22 kJ/mol (lutetium) | lowest | 5.5 kJ/mol (cerium) | distribution | molar heat of vaporization | median | 290 kJ/mol | highest | 415 kJ/mol (lutetium) | lowest | 160 kJ/mol (ytterbium) | distribution | specific heat at STP | median | 186 J/(kg K) | highest | 567 J/(kg K) (scandium) | lowest | 154 J/(kg K) (ytterbium and lutetium) | distribution | Néel point | median | 90.5 K | highest | 230 K (terbium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)