Input interpretation
beryllium cation
Structure diagram

Structure diagram
General properties

formula | Be^(2+) net ionic charge | +2 alternate names | beryllium(II) | beryllium(II) cation | beryllium(2+)
Ionic radii

beryllium cation state | radius 4-coordinate | 27 pm 6-coordinate | 45 pm
Units

Other properties

ion class | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions common sources of ion | beryllium sulfate tetrahydrate (1 eq) | beryllium chloride (1 eq) | beryllium acetylacetonate (1 eq)
Thermodynamic properties
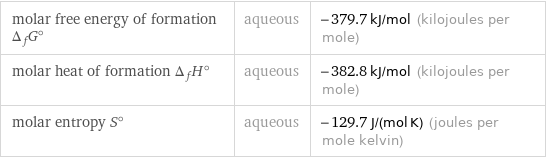
molar free energy of formation Δ_fG° | aqueous | -379.7 kJ/mol (kilojoules per mole) molar heat of formation Δ_fH° | aqueous | -382.8 kJ/mol (kilojoules per mole) molar entropy S° | aqueous | -129.7 J/(mol K) (joules per mole kelvin)