Input interpretation

alkaline earth metals | atomic volume
Summary
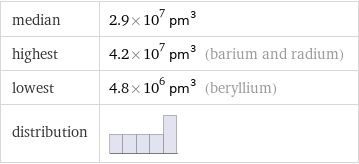
median | 2.9×10^7 pm^3 highest | 4.2×10^7 pm^3 (barium and radium) lowest | 4.8×10^6 pm^3 (beryllium) distribution |
Units

Ranked values
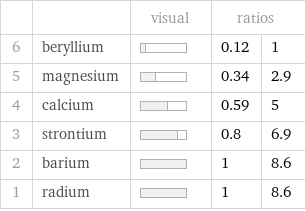
| | visual | ratios | 6 | beryllium | | 0.12 | 1 5 | magnesium | | 0.34 | 2.9 4 | calcium | | 0.59 | 5 3 | strontium | | 0.8 | 6.9 2 | barium | | 1 | 8.6 1 | radium | | 1 | 8.6
Plot
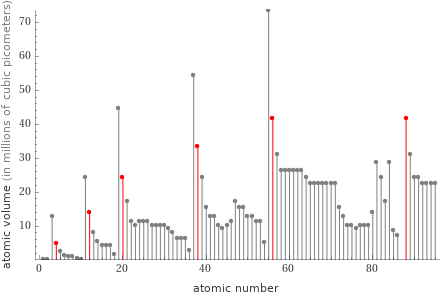
Plot
Periodic table location
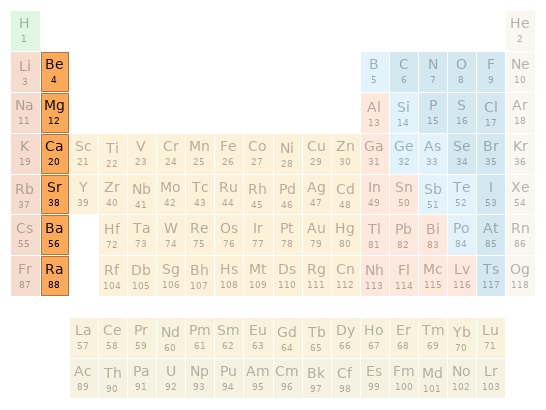
Periodic table location
Atomic radii properties
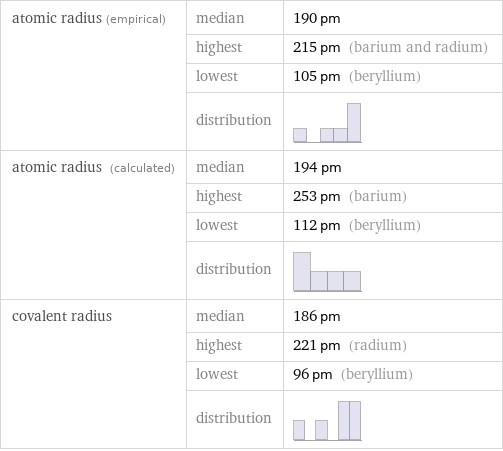
atomic radius (empirical) | median | 190 pm | highest | 215 pm (barium and radium) | lowest | 105 pm (beryllium) | distribution | atomic radius (calculated) | median | 194 pm | highest | 253 pm (barium) | lowest | 112 pm (beryllium) | distribution | covalent radius | median | 186 pm | highest | 221 pm (radium) | lowest | 96 pm (beryllium) | distribution |
Units

Atomic volume rankings
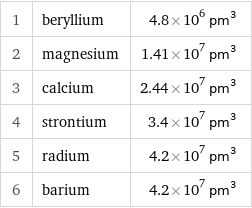
1 | beryllium | 4.8×10^6 pm^3 2 | magnesium | 1.41×10^7 pm^3 3 | calcium | 2.44×10^7 pm^3 4 | strontium | 3.4×10^7 pm^3 5 | radium | 4.2×10^7 pm^3 6 | barium | 4.2×10^7 pm^3
Unit conversions for median atomic volume 2.9×10^7 pm^3
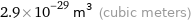
2.9×10^-29 m^3 (cubic meters)
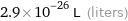
2.9×10^-26 L (liters)
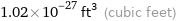
1.02×10^-27 ft^3 (cubic feet)
Corresponding quantities

Radius r of a sphere from V = 4πr^3/3: | 191 pm (picometers) | 7.5×10^-9 inches

Edge length a of a cube from V = a^3: | 307 pm (picometers) | 1.2×10^-8 inches