Input interpretation
hydrogen bromide | perchloric acid
Chemical names and formulas
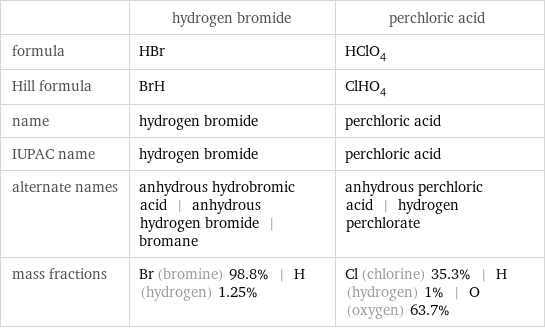
| hydrogen bromide | perchloric acid formula | HBr | HClO_4 Hill formula | BrH | ClHO_4 name | hydrogen bromide | perchloric acid IUPAC name | hydrogen bromide | perchloric acid alternate names | anhydrous hydrobromic acid | anhydrous hydrogen bromide | bromane | anhydrous perchloric acid | hydrogen perchlorate mass fractions | Br (bromine) 98.8% | H (hydrogen) 1.25% | Cl (chlorine) 35.3% | H (hydrogen) 1% | O (oxygen) 63.7%
Structure diagrams
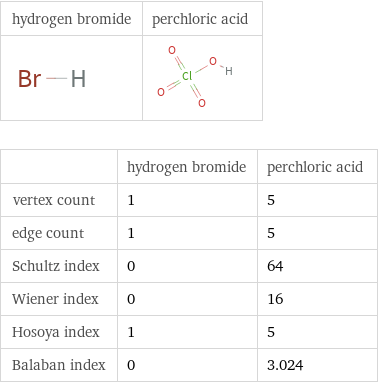
| hydrogen bromide | perchloric acid vertex count | 1 | 5 edge count | 1 | 5 Schultz index | 0 | 64 Wiener index | 0 | 16 Hosoya index | 1 | 5 Balaban index | 0 | 3.024
3D structure
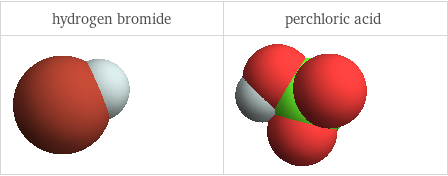
3D structure
Basic properties
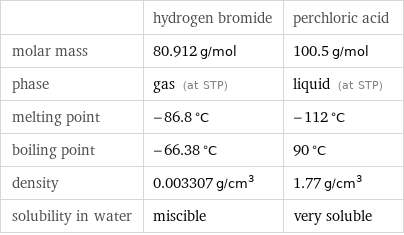
| hydrogen bromide | perchloric acid molar mass | 80.912 g/mol | 100.5 g/mol phase | gas (at STP) | liquid (at STP) melting point | -86.8 °C | -112 °C boiling point | -66.38 °C | 90 °C density | 0.003307 g/cm^3 | 1.77 g/cm^3 solubility in water | miscible | very soluble
Units

Gas properties
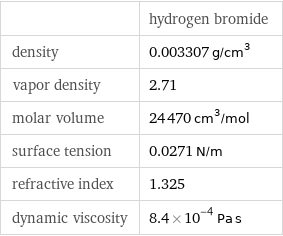
| hydrogen bromide density | 0.003307 g/cm^3 vapor density | 2.71 molar volume | 24470 cm^3/mol surface tension | 0.0271 N/m refractive index | 1.325 dynamic viscosity | 8.4×10^-4 Pa s
Units

Liquid properties
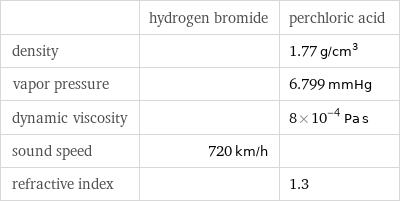
| hydrogen bromide | perchloric acid density | | 1.77 g/cm^3 vapor pressure | | 6.799 mmHg dynamic viscosity | | 8×10^-4 Pa s sound speed | 720 km/h | refractive index | | 1.3
Units
Thermodynamic properties
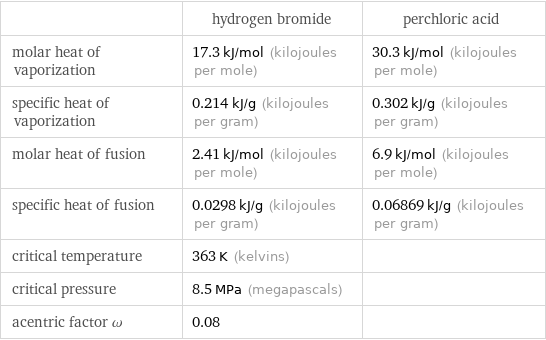
| hydrogen bromide | perchloric acid molar heat of vaporization | 17.3 kJ/mol (kilojoules per mole) | 30.3 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.214 kJ/g (kilojoules per gram) | 0.302 kJ/g (kilojoules per gram) molar heat of fusion | 2.41 kJ/mol (kilojoules per mole) | 6.9 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0298 kJ/g (kilojoules per gram) | 0.06869 kJ/g (kilojoules per gram) critical temperature | 363 K (kelvins) | critical pressure | 8.5 MPa (megapascals) | acentric factor ω | 0.08 |
Chemical identifiers
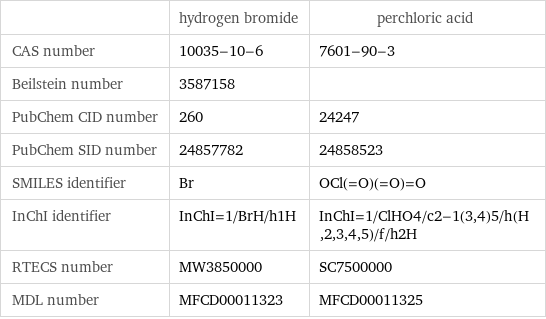
| hydrogen bromide | perchloric acid CAS number | 10035-10-6 | 7601-90-3 Beilstein number | 3587158 | PubChem CID number | 260 | 24247 PubChem SID number | 24857782 | 24858523 SMILES identifier | Br | OCl(=O)(=O)=O InChI identifier | InChI=1/BrH/h1H | InChI=1/ClHO4/c2-1(3, 4)5/h(H, 2, 3, 4, 5)/f/h2H RTECS number | MW3850000 | SC7500000 MDL number | MFCD00011323 | MFCD00011325
NFPA label

NFPA label
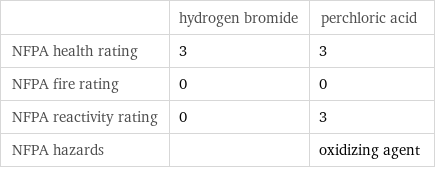
| hydrogen bromide | perchloric acid NFPA health rating | 3 | 3 NFPA fire rating | 0 | 0 NFPA reactivity rating | 0 | 3 NFPA hazards | | oxidizing agent
Toxicity properties

| hydrogen bromide | perchloric acid odor | | odorless short-term exposure limit | 10 mg/m^3 |
Units
