Input interpretation
elements | covalent radius
Summary
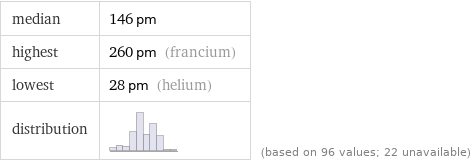
median | 146 pm highest | 260 pm (francium) lowest | 28 pm (helium) distribution | | (based on 96 values; 22 unavailable)
Entities with missing values

berkelium | californium | einsteinium | fermium | mendelevium | nobelium | lawrencium | rutherfordium | dubnium | seaborgium | ... (total: 22)
Plot
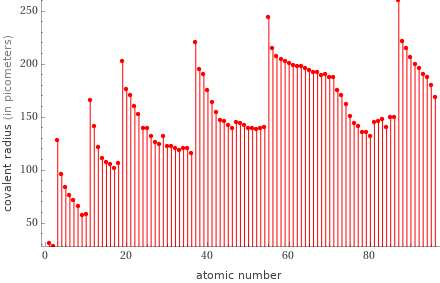
Plot
Periodic table location
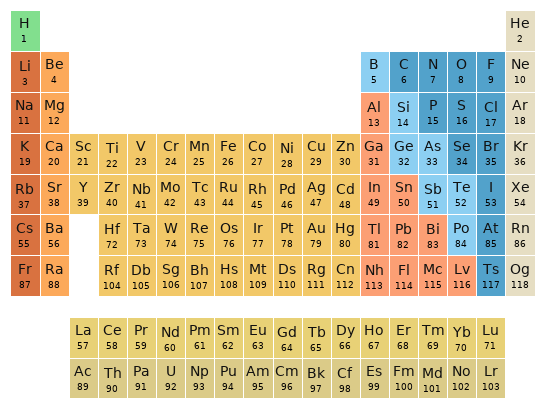
Periodic table location
Atomic radii properties
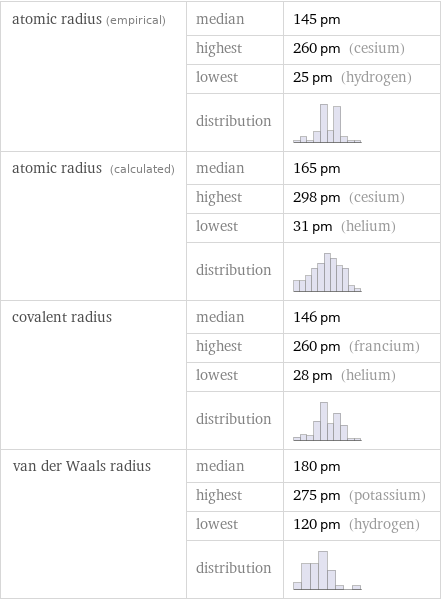
atomic radius (empirical) | median | 145 pm | highest | 260 pm (cesium) | lowest | 25 pm (hydrogen) | distribution | atomic radius (calculated) | median | 165 pm | highest | 298 pm (cesium) | lowest | 31 pm (helium) | distribution | covalent radius | median | 146 pm | highest | 260 pm (francium) | lowest | 28 pm (helium) | distribution | van der Waals radius | median | 180 pm | highest | 275 pm (potassium) | lowest | 120 pm (hydrogen) | distribution |
Units

Distribution plots
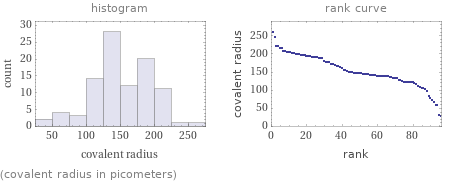
(covalent radius in picometers)
Covalent radius rankings
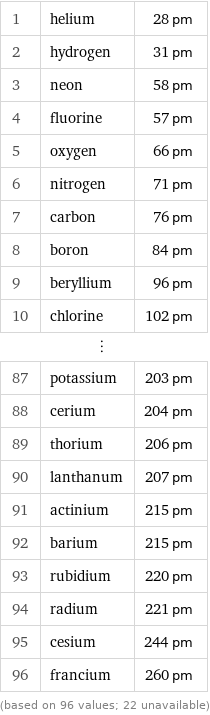
1 | helium | 28 pm 2 | hydrogen | 31 pm 3 | neon | 58 pm 4 | fluorine | 57 pm 5 | oxygen | 66 pm 6 | nitrogen | 71 pm 7 | carbon | 76 pm 8 | boron | 84 pm 9 | beryllium | 96 pm 10 | chlorine | 102 pm ⋮ | | 87 | potassium | 203 pm 88 | cerium | 204 pm 89 | thorium | 206 pm 90 | lanthanum | 207 pm 91 | actinium | 215 pm 92 | barium | 215 pm 93 | rubidium | 220 pm 94 | radium | 221 pm 95 | cesium | 244 pm 96 | francium | 260 pm (based on 96 values; 22 unavailable)
Unit conversions for median covalent radius 146 pm
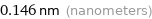
0.146 nm (nanometers)
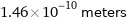
1.46×10^-10 meters
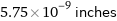
5.75×10^-9 inches
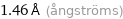
1.46 Å (ångströms)
Comparisons for median covalent radius 146 pm
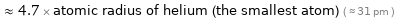
≈ 4.7 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
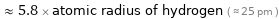
≈ 5.8 × atomic radius of hydrogen ( ≈ 25 pm )