Input interpretation
1 L of D-tyrosine
Basic properties for 1 L
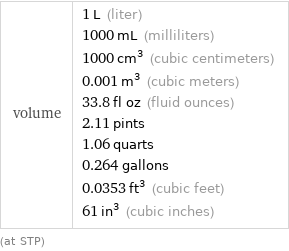
volume | 1 L (liter) 1000 mL (milliliters) 1000 cm^3 (cubic centimeters) 0.001 m^3 (cubic meters) 33.8 fl oz (fluid ounces) 2.11 pints 1.06 quarts 0.264 gallons 0.0353 ft^3 (cubic feet) 61 in^3 (cubic inches) (at STP)
Corresponding quantities

sphere radius | 6.204 cm (centimeters) side of a cube | 10 cm (centimeters)
Lewis structure
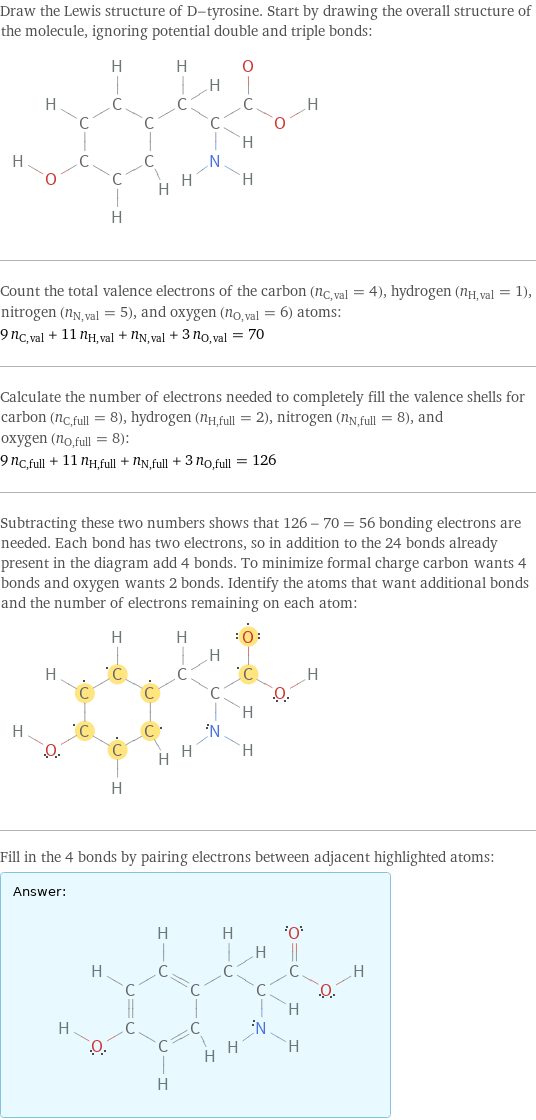
Draw the Lewis structure of D-tyrosine. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 9 n_C, val + 11 n_H, val + n_N, val + 3 n_O, val = 70 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 9 n_C, full + 11 n_H, full + n_N, full + 3 n_O, full = 126 Subtracting these two numbers shows that 126 - 70 = 56 bonding electrons are needed. Each bond has two electrons, so in addition to the 24 bonds already present in the diagram add 4 bonds. To minimize formal charge carbon wants 4 bonds and oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 4 bonds by pairing electrons between adjacent highlighted atoms: Answer: | |
Chemical names and formulas
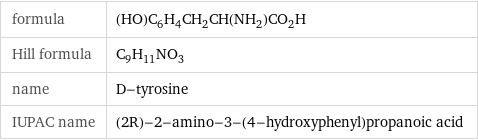
formula | (HO)C_6H_4CH_2CH(NH_2)CO_2H Hill formula | C_9H_11NO_3 name | D-tyrosine IUPAC name | (2R)-2-amino-3-(4-hydroxyphenyl)propanoic acid
Substance properties
molar mass | 181.19 g/mol phase | solid (at STP) melting point | 300 °C boiling point | 351 °C solubility in water | very soluble
Units
