Input interpretation
quarter-normal saline
Composition

sodium chloride | 2.2 g/L (grams per liter)
Solution properties
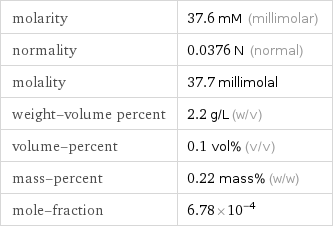
molarity | 37.6 mM (millimolar) normality | 0.0376 N (normal) molality | 37.7 millimolal weight-volume percent | 2.2 g/L (w/v) volume-percent | 0.1 vol% (v/v) mass-percent | 0.22 mass% (w/w) mole-fraction | 6.78×10^-4
Solute properties per 100 mL
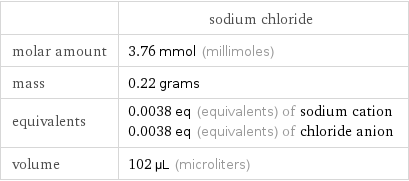
| sodium chloride molar amount | 3.76 mmol (millimoles) mass | 0.22 grams equivalents | 0.0038 eq (equivalents) of sodium cation 0.0038 eq (equivalents) of chloride anion volume | 102 µL (microliters)
Ionic species
Cations
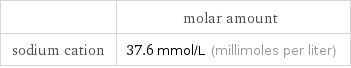
| molar amount sodium cation | 37.6 mmol/L (millimoles per liter)
Anions
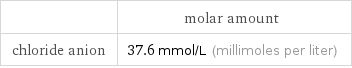
| molar amount chloride anion | 37.6 mmol/L (millimoles per liter)
Solvent properties per 100 mL
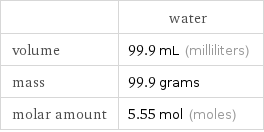
| water volume | 99.9 mL (milliliters) mass | 99.9 grams molar amount | 5.55 mol (moles)
Structure diagrams
Structure diagrams
Substance properties
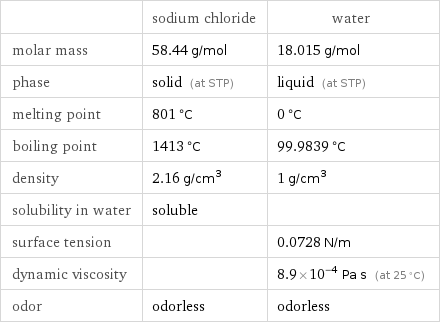
| sodium chloride | water molar mass | 58.44 g/mol | 18.015 g/mol phase | solid (at STP) | liquid (at STP) melting point | 801 °C | 0 °C boiling point | 1413 °C | 99.9839 °C density | 2.16 g/cm^3 | 1 g/cm^3 solubility in water | soluble | surface tension | | 0.0728 N/m dynamic viscosity | | 8.9×10^-4 Pa s (at 25 °C) odor | odorless | odorless
Units
