Input interpretation
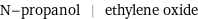
N-propanol | ethylene oxide
Chemical names and formulas
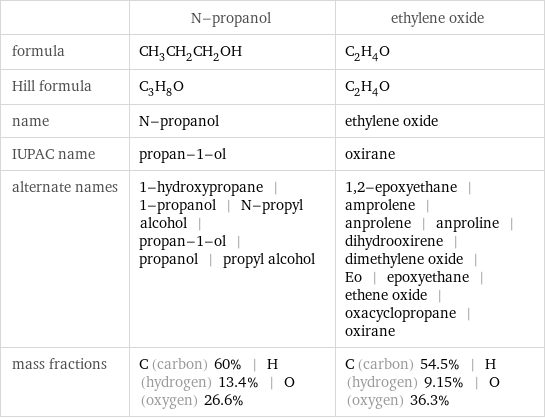
| N-propanol | ethylene oxide formula | CH_3CH_2CH_2OH | C_2H_4O Hill formula | C_3H_8O | C_2H_4O name | N-propanol | ethylene oxide IUPAC name | propan-1-ol | oxirane alternate names | 1-hydroxypropane | 1-propanol | N-propyl alcohol | propan-1-ol | propanol | propyl alcohol | 1, 2-epoxyethane | amprolene | anprolene | anproline | dihydrooxirene | dimethylene oxide | Eo | epoxyethane | ethene oxide | oxacyclopropane | oxirane mass fractions | C (carbon) 60% | H (hydrogen) 13.4% | O (oxygen) 26.6% | C (carbon) 54.5% | H (hydrogen) 9.15% | O (oxygen) 36.3%
Structure diagrams
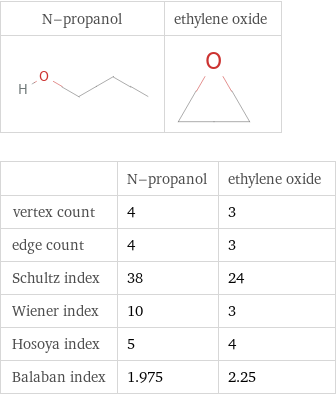
| N-propanol | ethylene oxide vertex count | 4 | 3 edge count | 4 | 3 Schultz index | 38 | 24 Wiener index | 10 | 3 Hosoya index | 5 | 4 Balaban index | 1.975 | 2.25
3D structure
3D structure
Basic properties
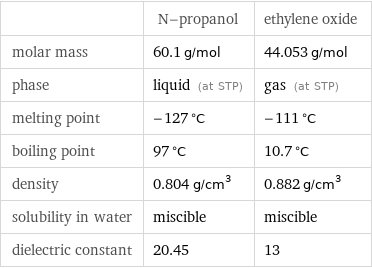
| N-propanol | ethylene oxide molar mass | 60.1 g/mol | 44.053 g/mol phase | liquid (at STP) | gas (at STP) melting point | -127 °C | -111 °C boiling point | 97 °C | 10.7 °C density | 0.804 g/cm^3 | 0.882 g/cm^3 solubility in water | miscible | miscible dielectric constant | 20.45 | 13
Hydrophobicity and permeability properties

| N-propanol predicted LogP hydrophobicity | 0.21 predicted LogS | 0.81
Gas properties
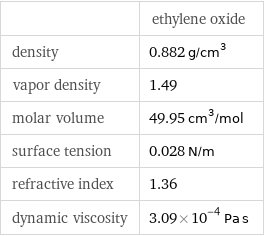
| ethylene oxide density | 0.882 g/cm^3 vapor density | 1.49 molar volume | 49.95 cm^3/mol surface tension | 0.028 N/m refractive index | 1.36 dynamic viscosity | 3.09×10^-4 Pa s
Units

Liquid properties
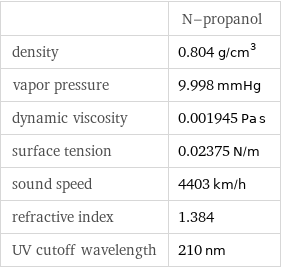
| N-propanol density | 0.804 g/cm^3 vapor pressure | 9.998 mmHg dynamic viscosity | 0.001945 Pa s surface tension | 0.02375 N/m sound speed | 4403 km/h refractive index | 1.384 UV cutoff wavelength | 210 nm
Units
Thermodynamic properties
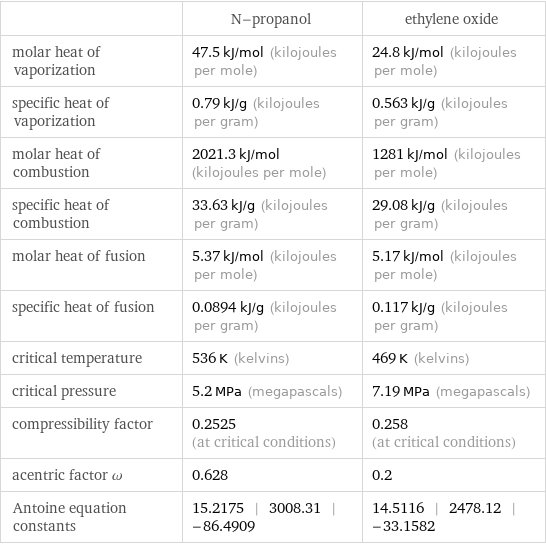
| N-propanol | ethylene oxide molar heat of vaporization | 47.5 kJ/mol (kilojoules per mole) | 24.8 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.79 kJ/g (kilojoules per gram) | 0.563 kJ/g (kilojoules per gram) molar heat of combustion | 2021.3 kJ/mol (kilojoules per mole) | 1281 kJ/mol (kilojoules per mole) specific heat of combustion | 33.63 kJ/g (kilojoules per gram) | 29.08 kJ/g (kilojoules per gram) molar heat of fusion | 5.37 kJ/mol (kilojoules per mole) | 5.17 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0894 kJ/g (kilojoules per gram) | 0.117 kJ/g (kilojoules per gram) critical temperature | 536 K (kelvins) | 469 K (kelvins) critical pressure | 5.2 MPa (megapascals) | 7.19 MPa (megapascals) compressibility factor | 0.2525 (at critical conditions) | 0.258 (at critical conditions) acentric factor ω | 0.628 | 0.2 Antoine equation constants | 15.2175 | 3008.31 | -86.4909 | 14.5116 | 2478.12 | -33.1582