Input interpretation
f-block elements | number of valence electrons
Summary

median | 2 e highest | 2 e (28 elements) lowest | 2 e (28 elements)
Units

Number of valence electrons rankings
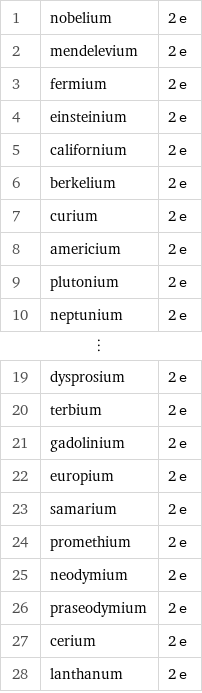
1 | nobelium | 2 e 2 | mendelevium | 2 e 3 | fermium | 2 e 4 | einsteinium | 2 e 5 | californium | 2 e 6 | berkelium | 2 e 7 | curium | 2 e 8 | americium | 2 e 9 | plutonium | 2 e 10 | neptunium | 2 e ⋮ | | 19 | dysprosium | 2 e 20 | terbium | 2 e 21 | gadolinium | 2 e 22 | europium | 2 e 23 | samarium | 2 e 24 | promethium | 2 e 25 | neodymium | 2 e 26 | praseodymium | 2 e 27 | cerium | 2 e 28 | lanthanum | 2 e
Corresponding quantity
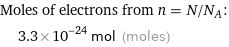
Moles of electrons from n = N/N_A: | 3.3×10^-24 mol (moles)