Input interpretation

complex ions | ionic radius
Summary

median | 265 pm highest | 342 pm (hexaiodoplatinate(IV) anion) lowest | 229 pm (hexaaquanickel(II) cation) distribution | | (based on 13 values; 7 unavailable)
Entities with missing values
tetrahydroxoberyllate anion | ferricyanide anion | ferrocyanide anion | dicyanoaurate(I) anion | diamminesilver(I) cation | dichloroaurate(I) anion | dibromoaurate(I) anion (total: 7)
Distribution plots
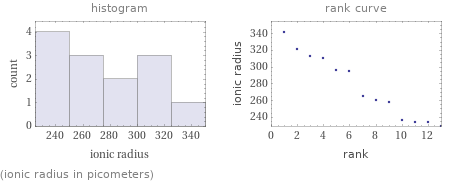
(ionic radius in picometers)
Ionic radius rankings
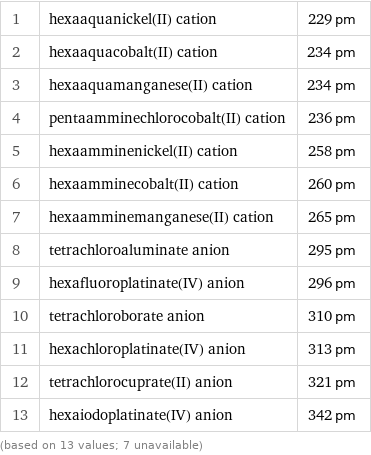
1 | hexaaquanickel(II) cation | 229 pm 2 | hexaaquacobalt(II) cation | 234 pm 3 | hexaaquamanganese(II) cation | 234 pm 4 | pentaamminechlorocobalt(II) cation | 236 pm 5 | hexaamminenickel(II) cation | 258 pm 6 | hexaamminecobalt(II) cation | 260 pm 7 | hexaamminemanganese(II) cation | 265 pm 8 | tetrachloroaluminate anion | 295 pm 9 | hexafluoroplatinate(IV) anion | 296 pm 10 | tetrachloroborate anion | 310 pm 11 | hexachloroplatinate(IV) anion | 313 pm 12 | tetrachlorocuprate(II) anion | 321 pm 13 | hexaiodoplatinate(IV) anion | 342 pm (based on 13 values; 7 unavailable)
Ionic radii
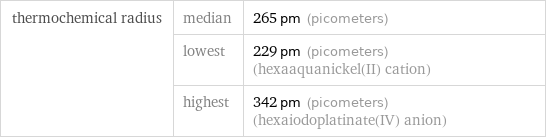
thermochemical radius | median | 265 pm (picometers) | lowest | 229 pm (picometers) (hexaaquanickel(II) cation) | highest | 342 pm (picometers) (hexaiodoplatinate(IV) anion)
Unit conversions for median ionic radius 265 pm

53/200 nm (nanometers)
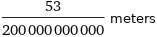
53/200000000000 meters
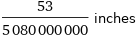
53/5080000000 inches

53/20 Å (ångströms)