Input interpretation
(s)-2-hydroxy-2-phenylpropionic acid;(s)-alpha-methylmandelic acid
Chemical names and formulas
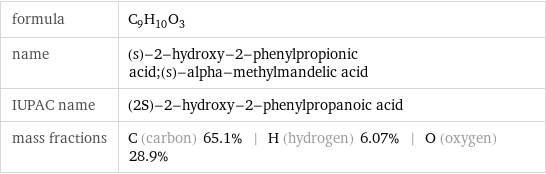
formula | C_9H_10O_3 name | (s)-2-hydroxy-2-phenylpropionic acid;(s)-alpha-methylmandelic acid IUPAC name | (2S)-2-hydroxy-2-phenylpropanoic acid mass fractions | C (carbon) 65.1% | H (hydrogen) 6.07% | O (oxygen) 28.9%
Lewis structure
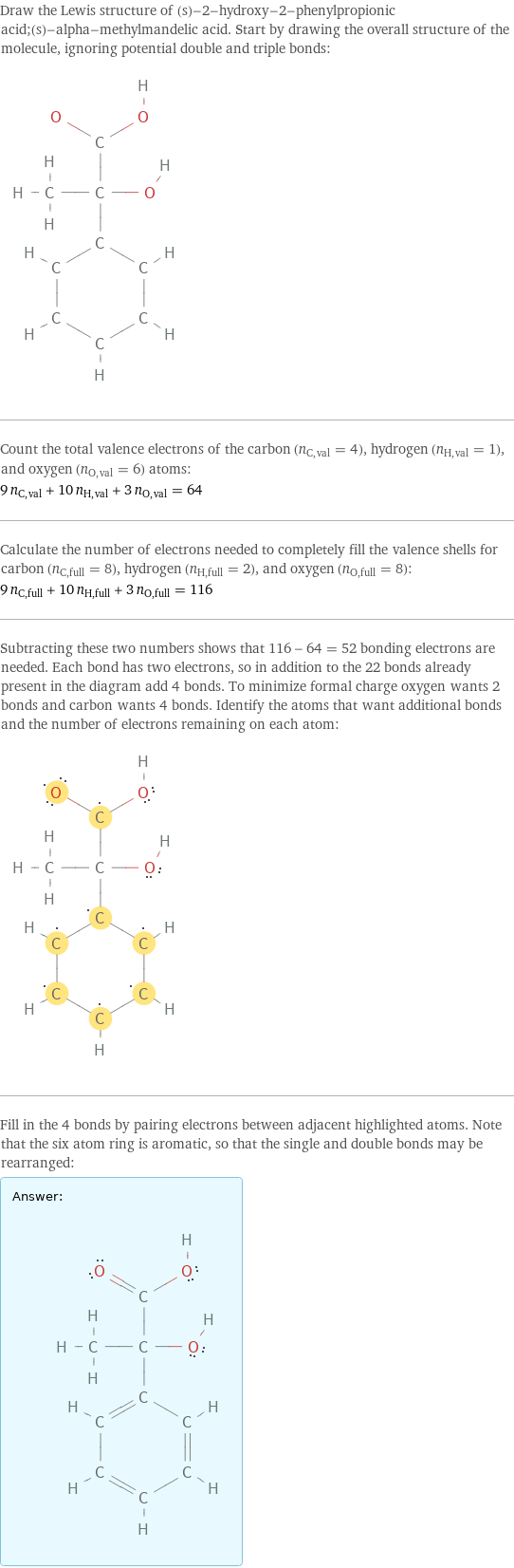
Draw the Lewis structure of (s)-2-hydroxy-2-phenylpropionic acid;(s)-alpha-methylmandelic acid. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 9 n_C, val + 10 n_H, val + 3 n_O, val = 64 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 9 n_C, full + 10 n_H, full + 3 n_O, full = 116 Subtracting these two numbers shows that 116 - 64 = 52 bonding electrons are needed. Each bond has two electrons, so in addition to the 22 bonds already present in the diagram add 4 bonds. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 4 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
molar mass | 166.18 g/mol phase | solid (at STP)
Units

Hydrophobicity and permeability properties

predicted LogP hydrophobicity | 1.07 predicted LogS | -0.93
Basic drug properties

approval status | experimental | small molecule
Chemical identifiers

PubChem CID number | 445144 PubChem SID number | 7890365 SMILES identifier | CC(C1=CC=CC=C1)(C(=O)O)O InChI identifier | InChI=1/C9H10O3/c1-9(12, 8(10)11)7-5-3-2-4-6-7/h2-6, 12H, 1H3, (H, 10, 11)/f/h10H InChI key | NWCHELUCVWSRRS-KZFATGLACX