Input interpretation
trimethylamine | ammonium hydroxide
Chemical names and formulas
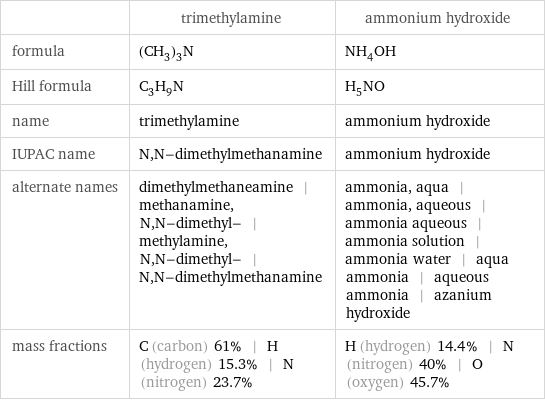
| trimethylamine | ammonium hydroxide formula | (CH_3)_3N | NH_4OH Hill formula | C_3H_9N | H_5NO name | trimethylamine | ammonium hydroxide IUPAC name | N, N-dimethylmethanamine | ammonium hydroxide alternate names | dimethylmethaneamine | methanamine, N, N-dimethyl- | methylamine, N, N-dimethyl- | N, N-dimethylmethanamine | ammonia, aqua | ammonia, aqueous | ammonia aqueous | ammonia solution | ammonia water | aqua ammonia | aqueous ammonia | azanium hydroxide mass fractions | C (carbon) 61% | H (hydrogen) 15.3% | N (nitrogen) 23.7% | H (hydrogen) 14.4% | N (nitrogen) 40% | O (oxygen) 45.7%
Structure diagrams
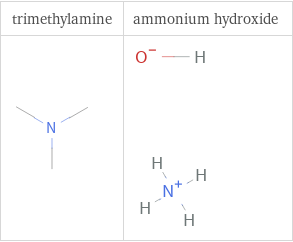
Structure diagrams
3D structure
3D structure
Basic properties
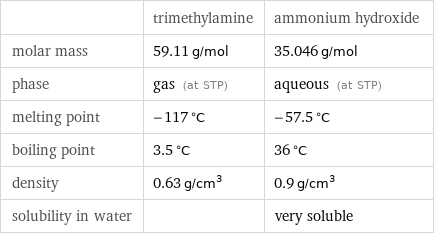
| trimethylamine | ammonium hydroxide molar mass | 59.11 g/mol | 35.046 g/mol phase | gas (at STP) | aqueous (at STP) melting point | -117 °C | -57.5 °C boiling point | 3.5 °C | 36 °C density | 0.63 g/cm^3 | 0.9 g/cm^3 solubility in water | | very soluble
Units

Gas properties
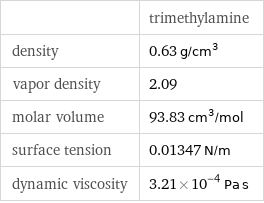
| trimethylamine density | 0.63 g/cm^3 vapor density | 2.09 molar volume | 93.83 cm^3/mol surface tension | 0.01347 N/m dynamic viscosity | 3.21×10^-4 Pa s
Units

Thermodynamic properties
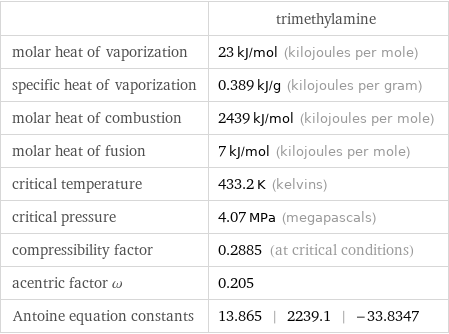
| trimethylamine molar heat of vaporization | 23 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.389 kJ/g (kilojoules per gram) molar heat of combustion | 2439 kJ/mol (kilojoules per mole) molar heat of fusion | 7 kJ/mol (kilojoules per mole) critical temperature | 433.2 K (kelvins) critical pressure | 4.07 MPa (megapascals) compressibility factor | 0.2885 (at critical conditions) acentric factor ω | 0.205 Antoine equation constants | 13.865 | 2239.1 | -33.8347