Input interpretation
silver telluride | nickel(II) ammonium chloride hexahydrate
Chemical names and formulas
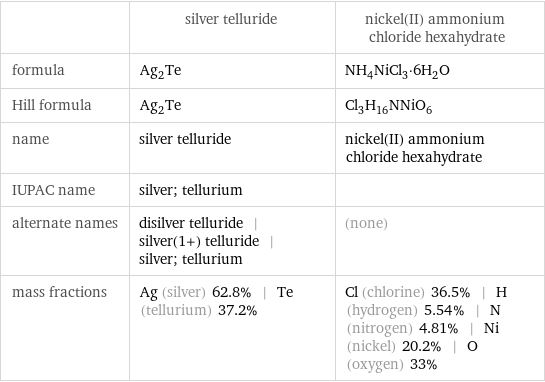
| silver telluride | nickel(II) ammonium chloride hexahydrate formula | Ag_2Te | NH_4NiCl_3·6H_2O Hill formula | Ag_2Te | Cl_3H_16NNiO_6 name | silver telluride | nickel(II) ammonium chloride hexahydrate IUPAC name | silver; tellurium | alternate names | disilver telluride | silver(1+) telluride | silver; tellurium | (none) mass fractions | Ag (silver) 62.8% | Te (tellurium) 37.2% | Cl (chlorine) 36.5% | H (hydrogen) 5.54% | N (nitrogen) 4.81% | Ni (nickel) 20.2% | O (oxygen) 33%
Structure diagrams
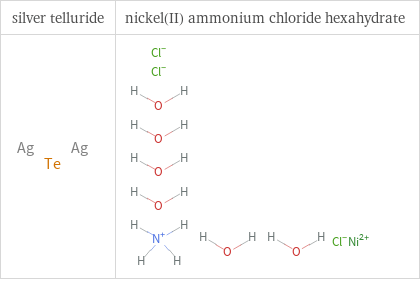
Structure diagrams
Basic properties
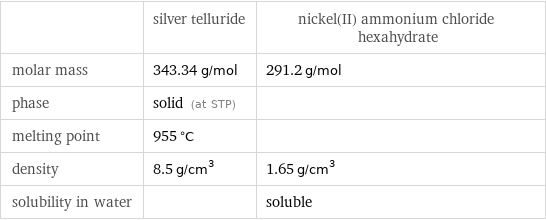
| silver telluride | nickel(II) ammonium chloride hexahydrate molar mass | 343.34 g/mol | 291.2 g/mol phase | solid (at STP) | melting point | 955 °C | density | 8.5 g/cm^3 | 1.65 g/cm^3 solubility in water | | soluble
Units
