Input interpretation
chloramine t | platinum
Chemical names and formulas
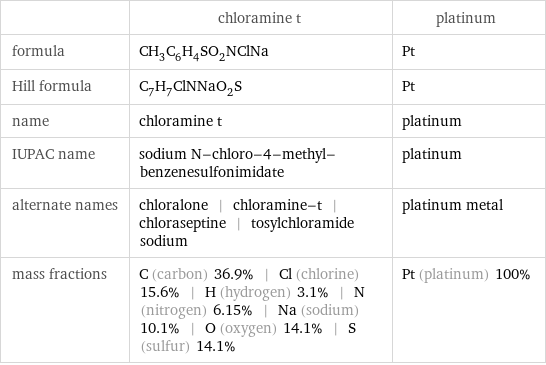
| chloramine t | platinum formula | CH_3C_6H_4SO_2NClNa | Pt Hill formula | C_7H_7ClNNaO_2S | Pt name | chloramine t | platinum IUPAC name | sodium N-chloro-4-methyl-benzenesulfonimidate | platinum alternate names | chloralone | chloramine-t | chloraseptine | tosylchloramide sodium | platinum metal mass fractions | C (carbon) 36.9% | Cl (chlorine) 15.6% | H (hydrogen) 3.1% | N (nitrogen) 6.15% | Na (sodium) 10.1% | O (oxygen) 14.1% | S (sulfur) 14.1% | Pt (platinum) 100%
Structure diagrams
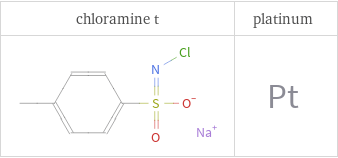
Structure diagrams
Basic properties
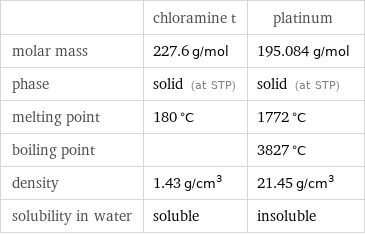
| chloramine t | platinum molar mass | 227.6 g/mol | 195.084 g/mol phase | solid (at STP) | solid (at STP) melting point | 180 °C | 1772 °C boiling point | | 3827 °C density | 1.43 g/cm^3 | 21.45 g/cm^3 solubility in water | soluble | insoluble
Units

Thermodynamic properties
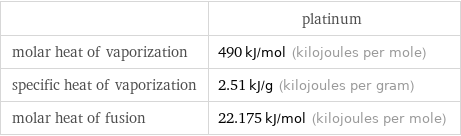
| platinum molar heat of vaporization | 490 kJ/mol (kilojoules per mole) specific heat of vaporization | 2.51 kJ/g (kilojoules per gram) molar heat of fusion | 22.175 kJ/mol (kilojoules per mole)