Input interpretation

pH
Equation
![pH = -log_10([H^+]) | [H^+] | hydrogen ion concentration pH | pH (assuming hydrogen ion concentration is in moles/liter)](../image_source/f14f24874a4095bb918c98dbfa6d3446.png)
pH = -log_10([H^+]) | [H^+] | hydrogen ion concentration pH | pH (assuming hydrogen ion concentration is in moles/liter)
Input value

pH | 1
Result
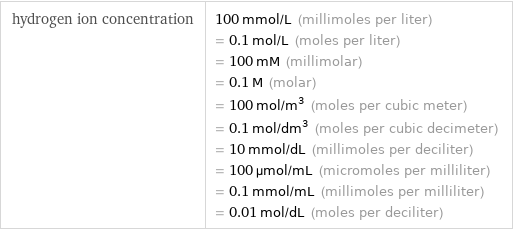
hydrogen ion concentration | 100 mmol/L (millimoles per liter) = 0.1 mol/L (moles per liter) = 100 mM (millimolar) = 0.1 M (molar) = 100 mol/m^3 (moles per cubic meter) = 0.1 mol/dm^3 (moles per cubic decimeter) = 10 mmol/dL (millimoles per deciliter) = 100 µmol/mL (micromoles per milliliter) = 0.1 mmol/mL (millimoles per milliliter) = 0.01 mol/dL (moles per deciliter)