Input interpretation
mercuric chloride | calcium iodide
Chemical names and formulas
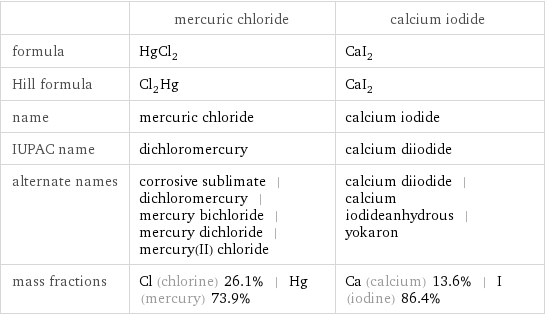
| mercuric chloride | calcium iodide formula | HgCl_2 | CaI_2 Hill formula | Cl_2Hg | CaI_2 name | mercuric chloride | calcium iodide IUPAC name | dichloromercury | calcium diiodide alternate names | corrosive sublimate | dichloromercury | mercury bichloride | mercury dichloride | mercury(II) chloride | calcium diiodide | calcium iodideanhydrous | yokaron mass fractions | Cl (chlorine) 26.1% | Hg (mercury) 73.9% | Ca (calcium) 13.6% | I (iodine) 86.4%
Structure diagrams

Structure diagrams
Basic properties
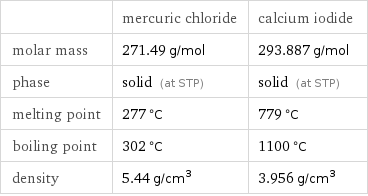
| mercuric chloride | calcium iodide molar mass | 271.49 g/mol | 293.887 g/mol phase | solid (at STP) | solid (at STP) melting point | 277 °C | 779 °C boiling point | 302 °C | 1100 °C density | 5.44 g/cm^3 | 3.956 g/cm^3
Units
