Input interpretation
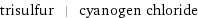
trisulfur | cyanogen chloride
Chemical names and formulas
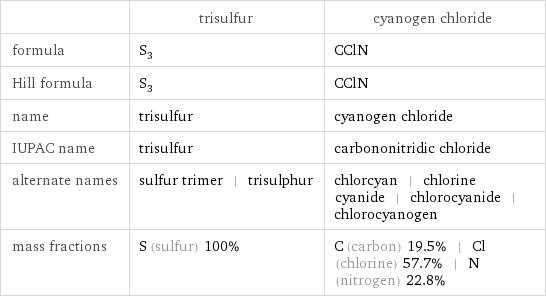
| trisulfur | cyanogen chloride formula | S_3 | CClN Hill formula | S_3 | CClN name | trisulfur | cyanogen chloride IUPAC name | trisulfur | carbononitridic chloride alternate names | sulfur trimer | trisulphur | chlorcyan | chlorine cyanide | chlorocyanide | chlorocyanogen mass fractions | S (sulfur) 100% | C (carbon) 19.5% | Cl (chlorine) 57.7% | N (nitrogen) 22.8%
Structure diagrams
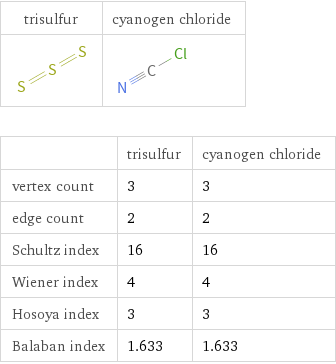
| trisulfur | cyanogen chloride vertex count | 3 | 3 edge count | 2 | 2 Schultz index | 16 | 16 Wiener index | 4 | 4 Hosoya index | 3 | 3 Balaban index | 1.633 | 1.633
3D structure

3D structure
Basic properties
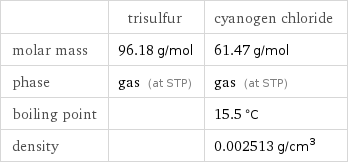
| trisulfur | cyanogen chloride molar mass | 96.18 g/mol | 61.47 g/mol phase | gas (at STP) | gas (at STP) boiling point | | 15.5 °C density | | 0.002513 g/cm^3
Units

Gas properties (at STP)
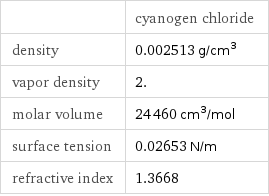
| cyanogen chloride density | 0.002513 g/cm^3 vapor density | 2. molar volume | 24460 cm^3/mol surface tension | 0.02653 N/m refractive index | 1.3668
Units
Thermodynamic properties
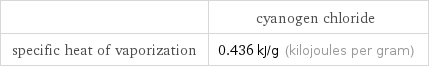
| cyanogen chloride specific heat of vaporization | 0.436 kJ/g (kilojoules per gram)