Input interpretation
silver bromide
Chemical names and formulas

formula | AgBr name | silver bromide IUPAC name | bromosilver alternate names | bromosilver | silver (1) bromide | silver brohide mass fractions | Ag (silver) 57.4% | Br (bromine) 42.6%
Structure diagram
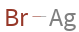
Structure diagram
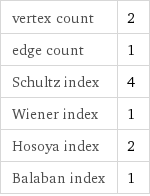
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
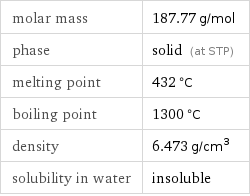
molar mass | 187.77 g/mol phase | solid (at STP) melting point | 432 °C boiling point | 1300 °C density | 6.473 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)
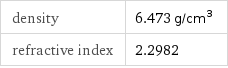
density | 6.473 g/cm^3 refractive index | 2.2982
Units

Thermodynamic properties
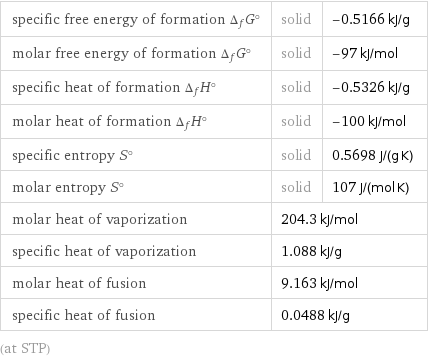
specific free energy of formation Δ_fG° | solid | -0.5166 kJ/g molar free energy of formation Δ_fG° | solid | -97 kJ/mol specific heat of formation Δ_fH° | solid | -0.5326 kJ/g molar heat of formation Δ_fH° | solid | -100 kJ/mol specific entropy S° | solid | 0.5698 J/(g K) molar entropy S° | solid | 107 J/(mol K) molar heat of vaporization | 204.3 kJ/mol | specific heat of vaporization | 1.088 kJ/g | molar heat of fusion | 9.163 kJ/mol | specific heat of fusion | 0.0488 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7785-23-1 PubChem CID number | 66199 PubChem SID number | 24853590 SMILES identifier | Br[Ag] InChI identifier | InChI=1/Ag.BrH/h;1H/q+1;/p-1/fAg.Br/h;1h/qm;-1 MDL number | MFCD00003398](../image_source/69cea892b1510f72a1c61feaa9976874.png)
CAS number | 7785-23-1 PubChem CID number | 66199 PubChem SID number | 24853590 SMILES identifier | Br[Ag] InChI identifier | InChI=1/Ag.BrH/h;1H/q+1;/p-1/fAg.Br/h;1h/qm;-1 MDL number | MFCD00003398