Input interpretation
hydrogen bromide
Chemical names and formulas
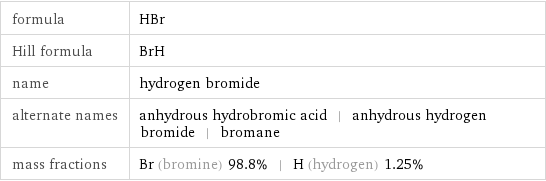
formula | HBr Hill formula | BrH name | hydrogen bromide alternate names | anhydrous hydrobromic acid | anhydrous hydrogen bromide | bromane mass fractions | Br (bromine) 98.8% | H (hydrogen) 1.25%
Lewis structure
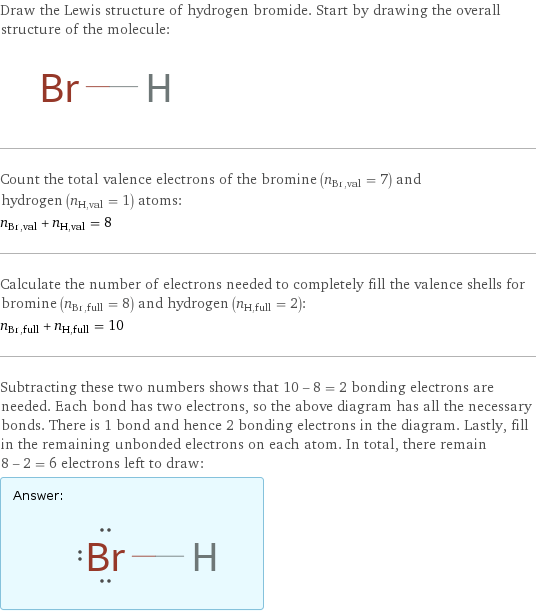
Draw the Lewis structure of hydrogen bromide. Start by drawing the overall structure of the molecule: Count the total valence electrons of the bromine (n_Br, val = 7) and hydrogen (n_H, val = 1) atoms: n_Br, val + n_H, val = 8 Calculate the number of electrons needed to completely fill the valence shells for bromine (n_Br, full = 8) and hydrogen (n_H, full = 2): n_Br, full + n_H, full = 10 Subtracting these two numbers shows that 10 - 8 = 2 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There is 1 bond and hence 2 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 8 - 2 = 6 electrons left to draw: Answer: | |
3D structure

3D structure
Basic properties
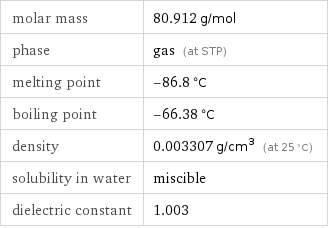
molar mass | 80.912 g/mol phase | gas (at STP) melting point | -86.8 °C boiling point | -66.38 °C density | 0.003307 g/cm^3 (at 25 °C) solubility in water | miscible dielectric constant | 1.003
Gas properties (at STP)
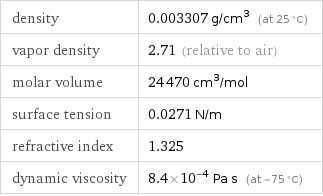
density | 0.003307 g/cm^3 (at 25 °C) vapor density | 2.71 (relative to air) molar volume | 24470 cm^3/mol surface tension | 0.0271 N/m refractive index | 1.325 dynamic viscosity | 8.4×10^-4 Pa s (at -75 °C)
Units

Liquid properties (-66.38 °C)

sound speed | 720 km/h (at 0 °C (degrees Celsius))
Units

Thermodynamic properties
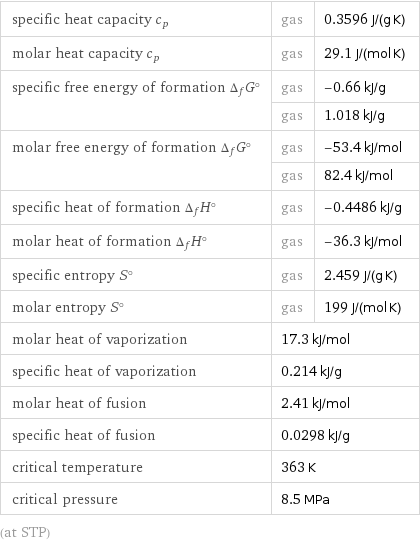
specific heat capacity c_p | gas | 0.3596 J/(g K) molar heat capacity c_p | gas | 29.1 J/(mol K) specific free energy of formation Δ_fG° | gas | -0.66 kJ/g | gas | 1.018 kJ/g molar free energy of formation Δ_fG° | gas | -53.4 kJ/mol | gas | 82.4 kJ/mol specific heat of formation Δ_fH° | gas | -0.4486 kJ/g molar heat of formation Δ_fH° | gas | -36.3 kJ/mol specific entropy S° | gas | 2.459 J/(g K) molar entropy S° | gas | 199 J/(mol K) molar heat of vaporization | 17.3 kJ/mol | specific heat of vaporization | 0.214 kJ/g | molar heat of fusion | 2.41 kJ/mol | specific heat of fusion | 0.0298 kJ/g | critical temperature | 363 K | critical pressure | 8.5 MPa | (at STP)
Chemical identifiers
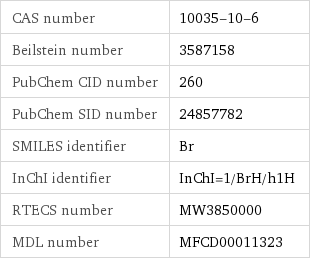
CAS number | 10035-10-6 Beilstein number | 3587158 PubChem CID number | 260 PubChem SID number | 24857782 SMILES identifier | Br InChI identifier | InChI=1/BrH/h1H RTECS number | MW3850000 MDL number | MFCD00011323
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

short-term exposure limit | 10 mg/m^3

long-term exposure limit | 10 mg/m^3 (over 8 hours) RTECS classes | other
Ion equivalents

H^+ (hydrogen cation) | 1 Br^- (bromide anion) | 1