Input interpretation
hydrogen carbonate anion
Lewis structure
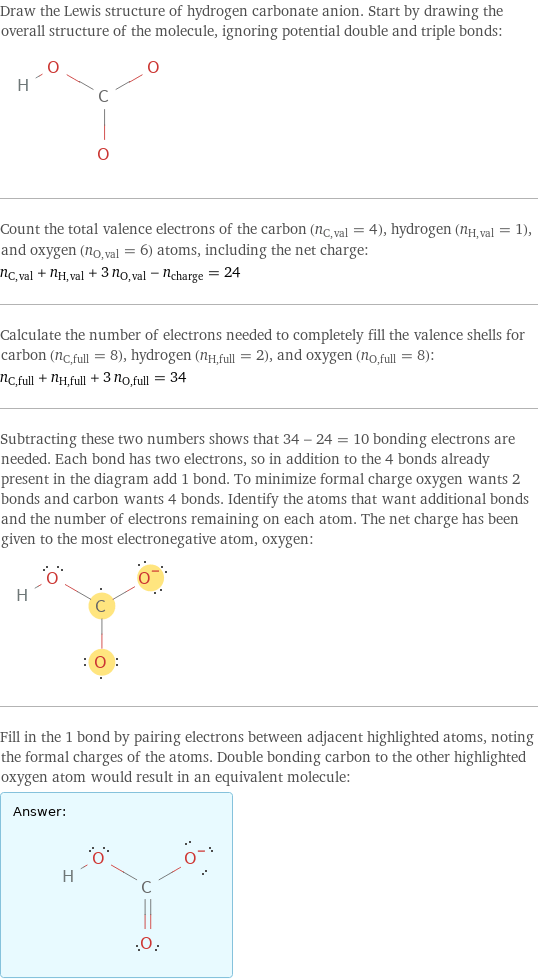
Draw the Lewis structure of hydrogen carbonate anion. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms, including the net charge: n_C, val + n_H, val + 3 n_O, val - n_charge = 24 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): n_C, full + n_H, full + 3 n_O, full = 34 Subtracting these two numbers shows that 34 - 24 = 10 bonding electrons are needed. Each bond has two electrons, so in addition to the 4 bonds already present in the diagram add 1 bond. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom. The net charge has been given to the most electronegative atom, oxygen: Fill in the 1 bond by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. Double bonding carbon to the other highlighted oxygen atom would result in an equivalent molecule: Answer: | |
General properties

formula | (HCO_3)^- net ionic charge | -1 alternate names | bicarbonate | hydrogen trioxocarbonate | hydrogen trioxidocarbonate | hydrogen carbonate | hydrogen carbonate(1-)
Ionic radius

thermochemical radius | 156 pm
Units

Other properties
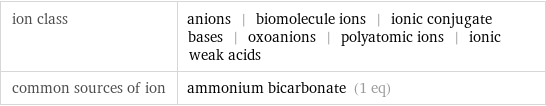
ion class | anions | biomolecule ions | ionic conjugate bases | oxoanions | polyatomic ions | ionic weak acids common sources of ion | ammonium bicarbonate (1 eq)
Thermodynamic properties
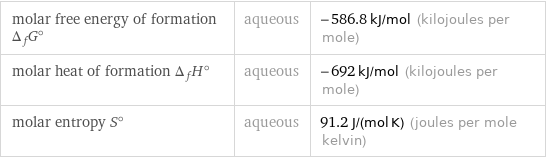
molar free energy of formation Δ_fG° | aqueous | -586.8 kJ/mol (kilojoules per mole) molar heat of formation Δ_fH° | aqueous | -692 kJ/mol (kilojoules per mole) molar entropy S° | aqueous | 91.2 J/(mol K) (joules per mole kelvin)