Input interpretation
D-(+)-glucose | orbital hybridization
Result
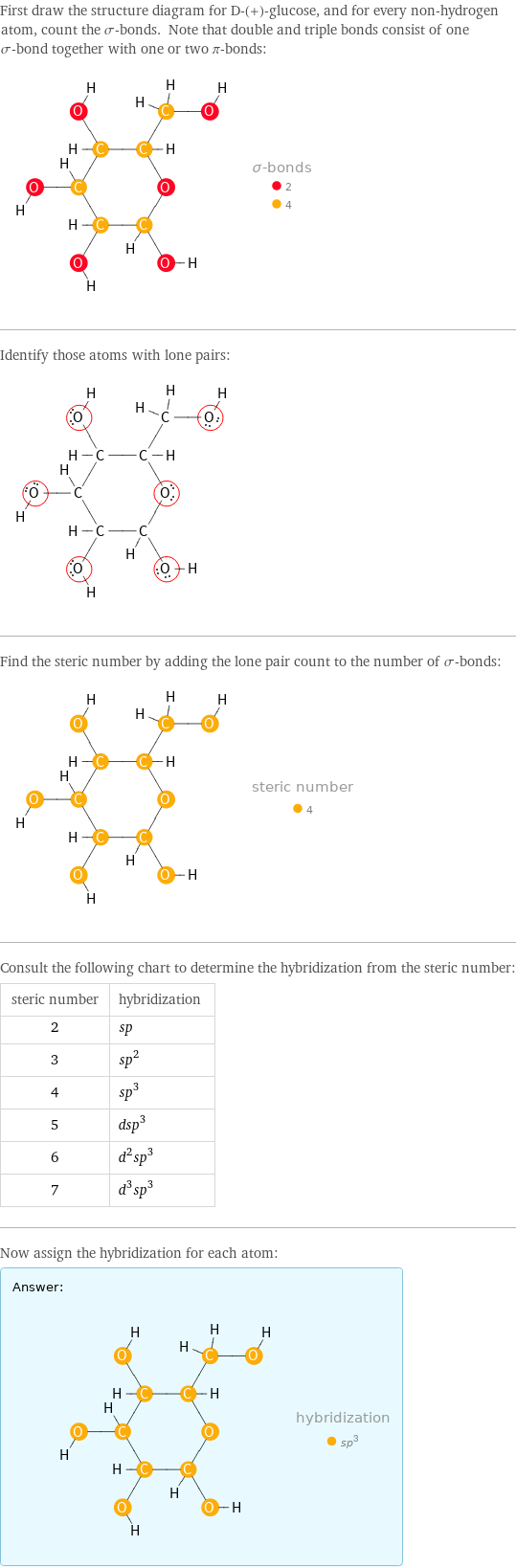
First draw the structure diagram for D-(+)-glucose, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Now assign the hybridization for each atom: Answer: | |
Chemical names and formulas
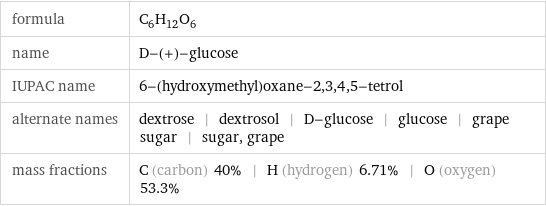
formula | C_6H_12O_6 name | D-(+)-glucose IUPAC name | 6-(hydroxymethyl)oxane-2, 3, 4, 5-tetrol alternate names | dextrose | dextrosol | D-glucose | glucose | grape sugar | sugar, grape mass fractions | C (carbon) 40% | H (hydrogen) 6.71% | O (oxygen) 53.3%