Input interpretation
silver | potassium metabisulfite
Chemical names and formulas
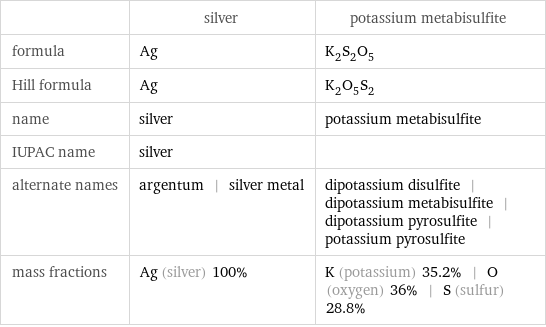
| silver | potassium metabisulfite formula | Ag | K_2S_2O_5 Hill formula | Ag | K_2O_5S_2 name | silver | potassium metabisulfite IUPAC name | silver | alternate names | argentum | silver metal | dipotassium disulfite | dipotassium metabisulfite | dipotassium pyrosulfite | potassium pyrosulfite mass fractions | Ag (silver) 100% | K (potassium) 35.2% | O (oxygen) 36% | S (sulfur) 28.8%
Structure diagrams
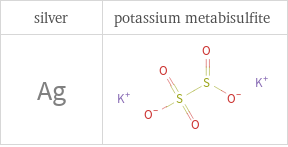
Structure diagrams
Basic properties
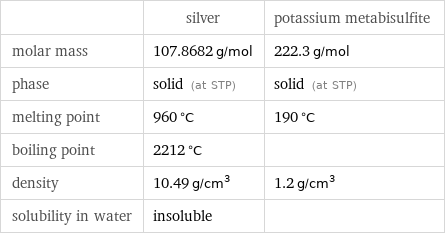
| silver | potassium metabisulfite molar mass | 107.8682 g/mol | 222.3 g/mol phase | solid (at STP) | solid (at STP) melting point | 960 °C | 190 °C boiling point | 2212 °C | density | 10.49 g/cm^3 | 1.2 g/cm^3 solubility in water | insoluble |
Units

Thermodynamic properties
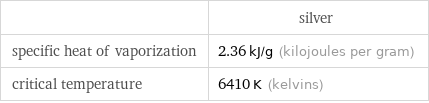
| silver specific heat of vaporization | 2.36 kJ/g (kilojoules per gram) critical temperature | 6410 K (kelvins)