Input interpretation
selenium oxide
Chemical names and formulas

formula | O_2Se name | selenium oxide alternate names | selenious anhydride | selenium monoxide mass fractions | O (oxygen) 28.8% | Se (selenium) 71.2%
Lewis structure

Draw the Lewis structure of selenium oxide. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the oxygen (n_O, val = 6) and selenium (n_Se, val = 6) atoms: 2 n_O, val + n_Se, val = 18 Calculate the number of electrons needed to completely fill the valence shells for oxygen (n_O, full = 8) and selenium (n_Se, full = 8): 2 n_O, full + n_Se, full = 24 Subtracting these two numbers shows that 24 - 18 = 6 bonding electrons are needed. Each bond has two electrons, so in addition to the 2 bonds already present in the diagram we expect to add 1 bond. To minimize formal charge oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Add 1 bond by pairing electrons between adjacent highlighted atoms. Additionally, atoms with large electronegativities can minimize their formal charge by forcing atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 2.55 (selenium) and 3.44 (oxygen). Because the electronegativity of selenium is smaller than the electronegativity of oxygen, expand the valence shell of selenium to 4 bonds. Therefore we add a total of 2 bonds to the diagram: Answer: | |
3D structure

3D structure
Basic properties

molar mass | 110.97 g/mol
Units

Thermodynamic properties
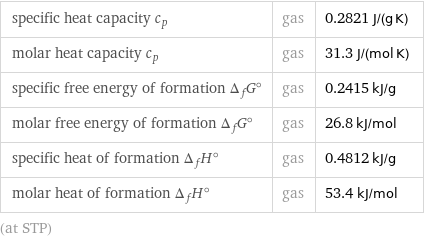
specific heat capacity c_p | gas | 0.2821 J/(g K) molar heat capacity c_p | gas | 31.3 J/(mol K) specific free energy of formation Δ_fG° | gas | 0.2415 kJ/g molar free energy of formation Δ_fG° | gas | 26.8 kJ/mol specific heat of formation Δ_fH° | gas | 0.4812 kJ/g molar heat of formation Δ_fH° | gas | 53.4 kJ/mol (at STP)
Chemical identifiers
![CAS number | 12640-89-0 PubChem CID number | 24007 SMILES identifier | O=[Se]=O InChI identifier | InChI=1/O2Se/c1-3-2 EU number | 235-738-4 Gmelin number | 25123 NSC number | 56753](../image_source/f84fa35c86442c1f4c059e84fd429289.png)
CAS number | 12640-89-0 PubChem CID number | 24007 SMILES identifier | O=[Se]=O InChI identifier | InChI=1/O2Se/c1-3-2 EU number | 235-738-4 Gmelin number | 25123 NSC number | 56753