Input interpretation
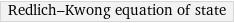
Redlich-Kwong equation of state
Equation

P = (R T)/(V_m - b) - a/(sqrt(T) V_m (V_m + b)) | P | pressure a | Redlich-Kwong constant a b | Redlich-Kwong constant b V_m | molar volume T | temperature R | molar gas constant (≈ 8.314 J/(mol K))
Units

Input values

Redlich-Kwong constant a | 0.336 Pa m^6 K^(1/2)/mol^2 (pascal meter to the sixth square root kelvins per mole squared) Redlich-Kwong constant b | 0.0011 L/mol (liters per mole) molar volume | 0.0245 L/mol (liters per mole) temperature | 298.2 K (kelvins)
Result

pressure | 74.91 MPa (megapascals) = 10865 psi (pounds-force per square inch) = 1.565×10^6 lbf/ft^2 (pounds-force per square foot) = 0.07491 GPa (gigapascals) = 7.491×10^7 Pa (pascals) = 739.3 atm (atmospheres) = 0.7491 kbar (kilobars)