Input interpretation

butane
Chemical names and formulas
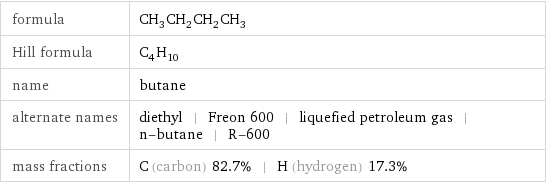
formula | CH_3CH_2CH_2CH_3 Hill formula | C_4H_10 name | butane alternate names | diethyl | Freon 600 | liquefied petroleum gas | n-butane | R-600 mass fractions | C (carbon) 82.7% | H (hydrogen) 17.3%
Lewis structure
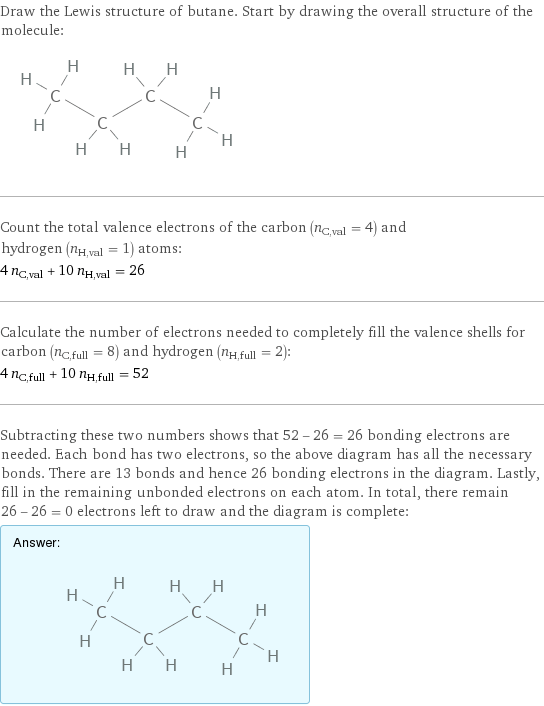
Draw the Lewis structure of butane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4) and hydrogen (n_H, val = 1) atoms: 4 n_C, val + 10 n_H, val = 26 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8) and hydrogen (n_H, full = 2): 4 n_C, full + 10 n_H, full = 52 Subtracting these two numbers shows that 52 - 26 = 26 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 13 bonds and hence 26 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 26 - 26 = 0 electrons left to draw and the diagram is complete: Answer: | |
3D structure
3D structure
Basic properties
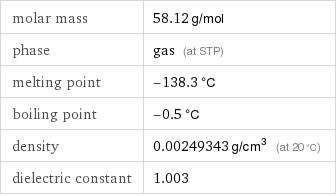
molar mass | 58.12 g/mol phase | gas (at STP) melting point | -138.3 °C boiling point | -0.5 °C density | 0.00249343 g/cm^3 (at 20 °C) dielectric constant | 1.003
Gas properties (at STP)
density | 0.00249343 g/cm^3 (at 20 °C) vapor density | 2.11 (relative to air) molar volume | 23311 cm^3/mol surface tension | 0.01487 N/m refractive index | 1.3326 dynamic viscosity | 7×10^-6 Pa s (at 25 °C)
Units

Thermodynamic properties
specific heat capacity c_p | liquid | 2.424 J/(g K) molar heat capacity c_p | liquid | 140.9 J/(mol K) specific heat of formation Δ_fH° | gas | -2.163 kJ/g molar heat of formation Δ_fH° | gas | -125.7 kJ/mol molar heat of vaporization | 24.7 kJ/mol | specific heat of vaporization | 0.425 kJ/g | molar heat of combustion | 2879 kJ/mol | specific heat of combustion | 49.53 kJ/g | molar heat of fusion | 4.66 kJ/mol | specific heat of fusion | 0.0802 kJ/g | thermal conductivity | 0.01607 W/(m K) | critical temperature | 426.3 K | critical pressure | 3.8 MPa | (at STP)
Phase diagram
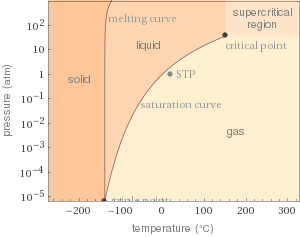
Phase diagram
Units

Basic drug properties
approval status | experimental | small molecule
Hydrophobicity and permeability properties

predicted LogP hydrophobicity | 2.81 predicted LogS | -1.91