Input interpretation

hexamethylmelamine | proton count
Result

Determine the number of protons in: hexamethylmelamine (C_9H_18N_6) Plan: • Use the summation: N_protons = sum_i N_i Z_i. • Determine the values of N_i and Z_i. • Compute the number of protons. Use the chemical formula, C_9H_18N_6, to count the number of atoms, N_i, for each element: | N_i C | 9 H | 18 N | 6 Look up the atomic number, Z_i, for each element in the periodic table. The number of protons per atom is equal to the atomic number: | N_i | Z_i C | 9 | 6 H | 18 | 1 N | 6 | 7 Multiply N_i by Z_i to compute the number of protons for each element: | N_i | Z_i | N_i Z_i C | 9 | 6 | 9 × 6 = 54 H | 18 | 1 | 18 × 1 = 18 N | 6 | 7 | 6 × 7 = 42 Sum the N_i Z_i terms to compute the number of protons, N_protons: Answer: | | N_protons = 54 + 18 + 42 = 114
Particle counts
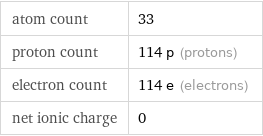
atom count | 33 proton count | 114 p (protons) electron count | 114 e (electrons) net ionic charge | 0
Corresponding quantity
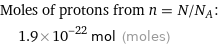
Moles of protons from n = N/N_A: | 1.9×10^-22 mol (moles)