Input interpretation
rhodium(III) chloride hydrate | vanadium boride
Chemical names and formulas
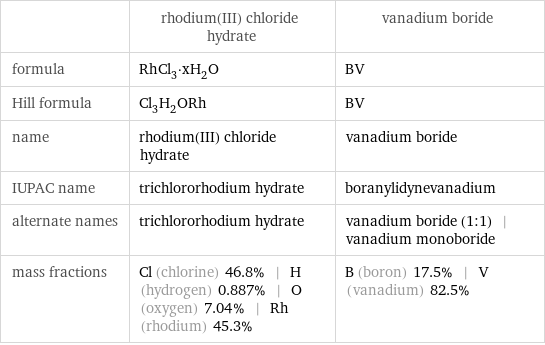
| rhodium(III) chloride hydrate | vanadium boride formula | RhCl_3·xH_2O | BV Hill formula | Cl_3H_2ORh | BV name | rhodium(III) chloride hydrate | vanadium boride IUPAC name | trichlororhodium hydrate | boranylidynevanadium alternate names | trichlororhodium hydrate | vanadium boride (1:1) | vanadium monoboride mass fractions | Cl (chlorine) 46.8% | H (hydrogen) 0.887% | O (oxygen) 7.04% | Rh (rhodium) 45.3% | B (boron) 17.5% | V (vanadium) 82.5%
Structure diagrams
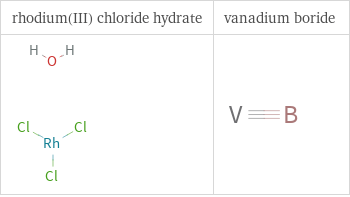
Structure diagrams
Basic properties

| rhodium(III) chloride hydrate | vanadium boride molar mass | 227.3 g/mol | 61.75 g/mol phase | solid (at STP) | solid (at STP) melting point | 100 °C | 2250 °C density | | 5.54 g/cm^3
Units
