Input interpretation
sodium sulfide
Chemical names and formulas

formula | Na_2S Hill formula | Na_2S_1 name | sodium sulfide alternate names | disodium bisulfide | disodium sulfanide | disodium sulfide | sodium monosulfide | sodium sulfide anhydrous mass fractions | Na (sodium) 58.9% | S (sulfur) 41.1%
Structure diagram
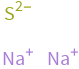
Structure diagram
Basic properties
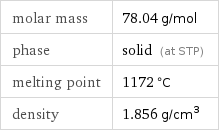
molar mass | 78.04 g/mol phase | solid (at STP) melting point | 1172 °C density | 1.856 g/cm^3
Units

Solid properties (at STP)

density | 1.856 g/cm^3
Units

Thermodynamic properties
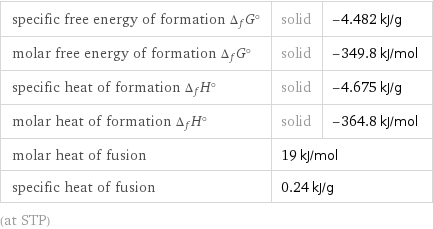
specific free energy of formation Δ_fG° | solid | -4.482 kJ/g molar free energy of formation Δ_fG° | solid | -349.8 kJ/mol specific heat of formation Δ_fH° | solid | -4.675 kJ/g molar heat of formation Δ_fH° | solid | -364.8 kJ/mol molar heat of fusion | 19 kJ/mol | specific heat of fusion | 0.24 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1313-82-2 SMILES identifier | [Na+].[Na+].[S-2] RTECS number | WE1905000 MDL number | MFCD00003498](../image_source/38fc3847aa0a03af288c77404bec6d37.png)
CAS number | 1313-82-2 SMILES identifier | [Na+].[Na+].[S-2] RTECS number | WE1905000 MDL number | MFCD00003498
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 1 NFPA reactivity rating | 1
Toxicity properties

RTECS classes | other