Input interpretation
calcium bromide
Chemical names and formulas

formula | CaBr_2 Hill formula | Br_2Ca name | calcium bromide IUPAC name | calcium dibromide alternate names | calcium bromide dihydrate | calcium dibromide mass fractions | Br (bromine) 79.9% | Ca (calcium) 20.1%
Structure diagram
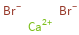
Structure diagram
Basic properties
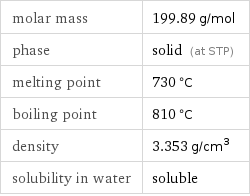
molar mass | 199.89 g/mol phase | solid (at STP) melting point | 730 °C boiling point | 810 °C density | 3.353 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)

density | 3.353 g/cm^3
Units

Thermodynamic properties
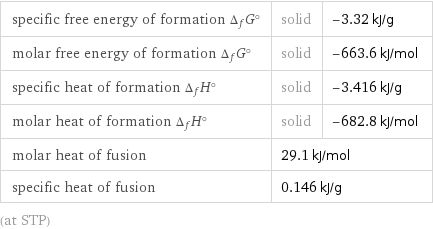
specific free energy of formation Δ_fG° | solid | -3.32 kJ/g molar free energy of formation Δ_fG° | solid | -663.6 kJ/mol specific heat of formation Δ_fH° | solid | -3.416 kJ/g molar heat of formation Δ_fH° | solid | -682.8 kJ/mol molar heat of fusion | 29.1 kJ/mol | specific heat of fusion | 0.146 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7789-41-5 PubChem CID number | 24608 SMILES identifier | [Ca+2].[Br-].[Br-] InChI identifier | InChI=1/2BrH.Ca/h2*1H;/q;;+2/p-2/f2Br.Ca/h2*1h;/q2*-1;m RTECS number | EV9328000 MDL number | MFCD00010902](../image_source/1443b7adad695f9074330d558c7ad08e.png)
CAS number | 7789-41-5 PubChem CID number | 24608 SMILES identifier | [Ca+2].[Br-].[Br-] InChI identifier | InChI=1/2BrH.Ca/h2*1H;/q;;+2/p-2/f2Br.Ca/h2*1h;/q2*-1;m RTECS number | EV9328000 MDL number | MFCD00010902
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | mutagen
Ion equivalents

Ca^(2+) (calcium cation) | 1 Br^- (bromide anion) | 2