Input interpretation

potassium hexacyanoferrate(III) | ferrous oxalate
Chemical names and formulas
![| potassium hexacyanoferrate(III) | ferrous oxalate formula | K_3Fe(CN)_6 | C_2FeO_4 Hill formula | C_6FeK_3N_6 | C_2FeO_4 name | potassium hexacyanoferrate(III) | ferrous oxalate IUPAC name | ferric tripotassium hexacyanide | iron(+2) cation; oxalate alternate names | ferric tripotassium hexacyanide | potassium ferricyanide | red prussiate | iron, [ethanedioato(2-)-O, O']- | iron(II) oxalate | iron protoxalate | oxalic acid, iron(2+) salt mass fractions | C (carbon) 21.9% | Fe (iron) 17% | K (potassium) 35.6% | N (nitrogen) 25.5% | C (carbon) 16.7% | Fe (iron) 38.8% | O (oxygen) 44.5%](../image_source/fb062cb8c5491ecb9d9e995401b6464f.png)
| potassium hexacyanoferrate(III) | ferrous oxalate formula | K_3Fe(CN)_6 | C_2FeO_4 Hill formula | C_6FeK_3N_6 | C_2FeO_4 name | potassium hexacyanoferrate(III) | ferrous oxalate IUPAC name | ferric tripotassium hexacyanide | iron(+2) cation; oxalate alternate names | ferric tripotassium hexacyanide | potassium ferricyanide | red prussiate | iron, [ethanedioato(2-)-O, O']- | iron(II) oxalate | iron protoxalate | oxalic acid, iron(2+) salt mass fractions | C (carbon) 21.9% | Fe (iron) 17% | K (potassium) 35.6% | N (nitrogen) 25.5% | C (carbon) 16.7% | Fe (iron) 38.8% | O (oxygen) 44.5%
Structure diagrams
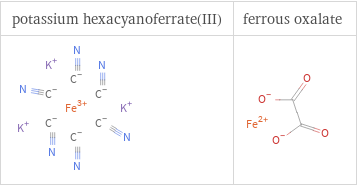
Structure diagrams
Basic properties

| potassium hexacyanoferrate(III) | ferrous oxalate molar mass | 329.25 g/mol | 143.86 g/mol density | 1.723 g/cm^3 | 2.3 g/cm^3 solubility in water | | slightly soluble
Units
