Input interpretation
manganese monoxide | tin(II) bromide
Chemical names and formulas
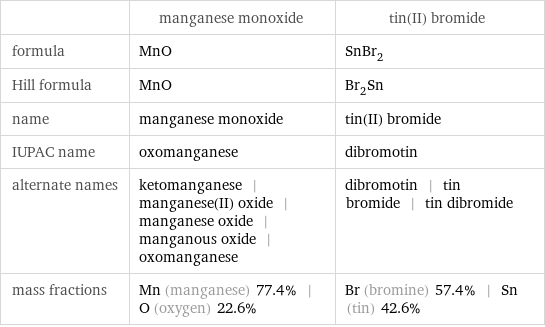
| manganese monoxide | tin(II) bromide formula | MnO | SnBr_2 Hill formula | MnO | Br_2Sn name | manganese monoxide | tin(II) bromide IUPAC name | oxomanganese | dibromotin alternate names | ketomanganese | manganese(II) oxide | manganese oxide | manganous oxide | oxomanganese | dibromotin | tin bromide | tin dibromide mass fractions | Mn (manganese) 77.4% | O (oxygen) 22.6% | Br (bromine) 57.4% | Sn (tin) 42.6%
Structure diagrams
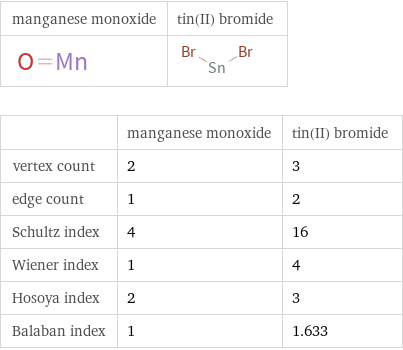
| manganese monoxide | tin(II) bromide vertex count | 2 | 3 edge count | 1 | 2 Schultz index | 4 | 16 Wiener index | 1 | 4 Hosoya index | 2 | 3 Balaban index | 1 | 1.633
Basic properties
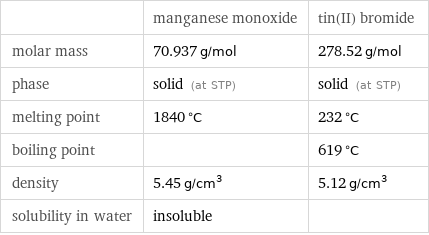
| manganese monoxide | tin(II) bromide molar mass | 70.937 g/mol | 278.52 g/mol phase | solid (at STP) | solid (at STP) melting point | 1840 °C | 232 °C boiling point | | 619 °C density | 5.45 g/cm^3 | 5.12 g/cm^3 solubility in water | insoluble |
Units

Thermodynamic properties
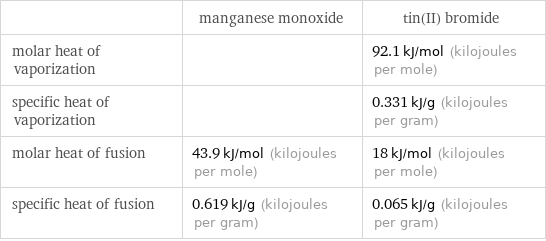
| manganese monoxide | tin(II) bromide molar heat of vaporization | | 92.1 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.331 kJ/g (kilojoules per gram) molar heat of fusion | 43.9 kJ/mol (kilojoules per mole) | 18 kJ/mol (kilojoules per mole) specific heat of fusion | 0.619 kJ/g (kilojoules per gram) | 0.065 kJ/g (kilojoules per gram)
Solid properties (at STP)

| manganese monoxide | tin(II) bromide density | 5.45 g/cm^3 | 5.12 g/cm^3
Units

Chemical identifiers
![| manganese monoxide | tin(II) bromide CAS number | 1344-43-0 | 10031-24-0 PubChem CID number | 14940 | 66224 PubChem SID number | 24863211 | 24858721 SMILES identifier | O=[Mn] | Br[Sn]Br](../image_source/3e13754b74eaad8688710f52246cf87c.png)
| manganese monoxide | tin(II) bromide CAS number | 1344-43-0 | 10031-24-0 PubChem CID number | 14940 | 66224 PubChem SID number | 24863211 | 24858721 SMILES identifier | O=[Mn] | Br[Sn]Br