Input interpretation

water | specific enthalpy | temperature | 400 K (kelvins) pressure | 40 MPa (megapascals)
Result
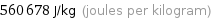
560678 J/kg (joules per kilogram)
Unit conversions
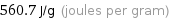
560.7 J/g (joules per gram)
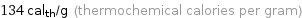
134 cal_th/g (thermochemical calories per gram)
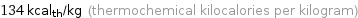
134 kcal_th/kg (thermochemical kilocalories per kilogram)
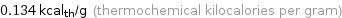
0.134 kcal_th/g (thermochemical kilocalories per gram)

241 BTU_IT/lb (IT British thermal units per pound)
Thermodynamic properties
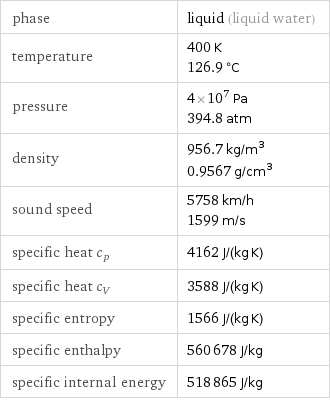
phase | liquid (liquid water) temperature | 400 K 126.9 °C pressure | 4×10^7 Pa 394.8 atm density | 956.7 kg/m^3 0.9567 g/cm^3 sound speed | 5758 km/h 1599 m/s specific heat c_p | 4162 J/(kg K) specific heat c_V | 3588 J/(kg K) specific entropy | 1566 J/(kg K) specific enthalpy | 560678 J/kg specific internal energy | 518865 J/kg
Phase diagram
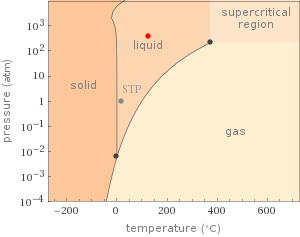
Phase diagram