Input interpretation

Mg magnesium + P red phosphorus ⟶ C_34H_32Mg_1N_4O_4 mg-protoporphyrin
Chemical names and formulas
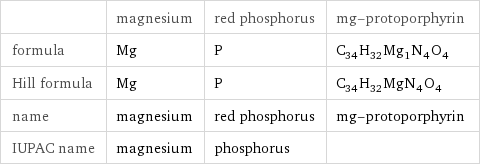
| magnesium | red phosphorus | mg-protoporphyrin formula | Mg | P | C_34H_32Mg_1N_4O_4 Hill formula | Mg | P | C_34H_32MgN_4O_4 name | magnesium | red phosphorus | mg-protoporphyrin IUPAC name | magnesium | phosphorus |
Substance properties
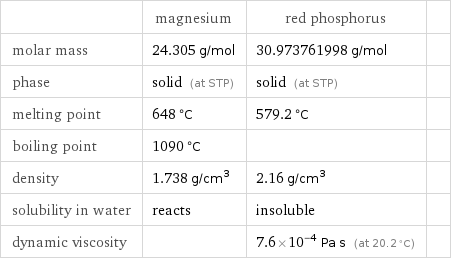
| magnesium | red phosphorus | molar mass | 24.305 g/mol | 30.973761998 g/mol | phase | solid (at STP) | solid (at STP) | melting point | 648 °C | 579.2 °C | boiling point | 1090 °C | | density | 1.738 g/cm^3 | 2.16 g/cm^3 | solubility in water | reacts | insoluble | dynamic viscosity | | 7.6×10^-4 Pa s (at 20.2 °C) |
Units
