Input interpretation
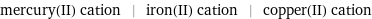
mercury(II) cation | iron(II) cation | copper(II) cation
Structure diagrams
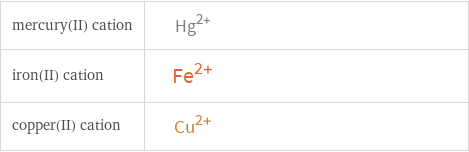
Structure diagrams
General properties
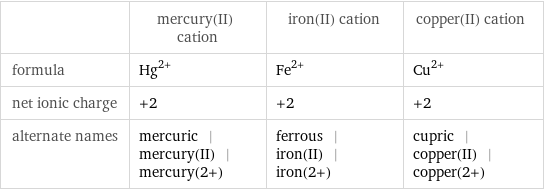
| mercury(II) cation | iron(II) cation | copper(II) cation formula | Hg^(2+) | Fe^(2+) | Cu^(2+) net ionic charge | +2 | +2 | +2 alternate names | mercuric | mercury(II) | mercury(2+) | ferrous | iron(II) | iron(2+) | cupric | copper(II) | copper(2+)
Ionic radii
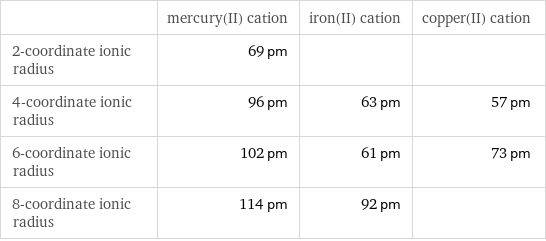
| mercury(II) cation | iron(II) cation | copper(II) cation 2-coordinate ionic radius | 69 pm | | 4-coordinate ionic radius | 96 pm | 63 pm | 57 pm 6-coordinate ionic radius | 102 pm | 61 pm | 73 pm 8-coordinate ionic radius | 114 pm | 92 pm |
Units

Other properties
| mercury(II) cation | iron(II) cation | copper(II) cation ion class | biomolecule ions | cations | d block ions | group 12 ions | mercury(II) ions | monatomic cations | transition metal ions | biomolecule ions | cations | d block ions | group 8 ions | iron(II) ions | monatomic cations | transition metal ions | biomolecule ions | cations | copper(II) ions | d block ions | group 11 ions | monatomic cations | transition metal ions common sources of ion | mercury dithizonate (1 eq) | mercury(I) fluoride (1 eq) | triirondodecacarbonyl (1 eq) | iron(II) sulfate hydrate (1 eq) | iron phthalocyanine (1 eq) | iron(II) molybdate (1 eq) | propyl astra blue (1 eq) | potassium cyanocuprate(I) (1 eq) | copper iron oxide (1 eq) | copper(II) tartrate hydrate (1 eq) | copper(II) sulfate pentahydrate (1 eq) | copper(II) sulfate hydrate (1 eq) | copper(II) pyrophosphate hydrate (2 eq) | copper(II) nitrate hydrate (2 eq) | copper(II) niobate (1 eq) | copper(II) molybdate (1 eq)