Input interpretation

lead ions | molar mass
Results
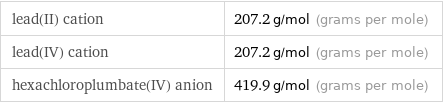
lead(II) cation | 207.2 g/mol (grams per mole) lead(IV) cation | 207.2 g/mol (grams per mole) hexachloroplumbate(IV) anion | 419.9 g/mol (grams per mole)

lead ions | molar mass | 207.2 g/mol (grams per mole) (median)
Ranked values

| | visual | ratios | 3 | lead(II) cation | | 0.4934 | 1 2 | lead(IV) cation | | 0.4934 | 1 1 | hexachloroplumbate(IV) anion | | 1 | 2.027
Unit conversion for median molar mass 207.2 g/mol
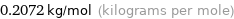
0.2072 kg/mol (kilograms per mole)
Comparisons for median molar mass 207.2 g/mol
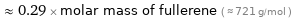
≈ 0.29 × molar mass of fullerene ( ≈ 721 g/mol )
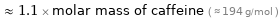
≈ 1.1 × molar mass of caffeine ( ≈ 194 g/mol )
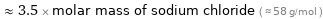
≈ 3.5 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
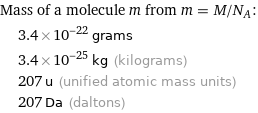
Mass of a molecule m from m = M/N_A: | 3.4×10^-22 grams | 3.4×10^-25 kg (kilograms) | 207 u (unified atomic mass units) | 207 Da (daltons)
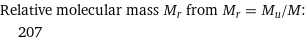
Relative molecular mass M_r from M_r = M_u/M: | 207