Input interpretation
ammonium cation | phosphate anion | nitrate anion
Structure diagrams
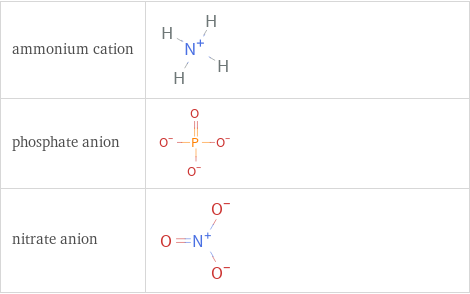
Structure diagrams
General properties
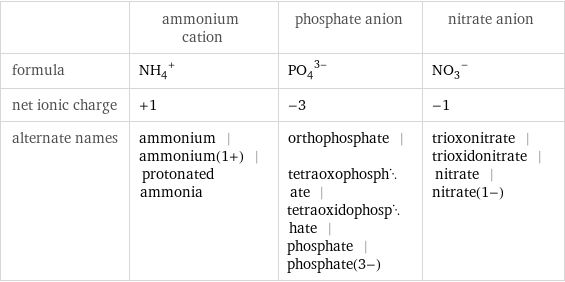
| ammonium cation | phosphate anion | nitrate anion formula | (NH_4)^+ | (PO_4)^(3-) | (NO_3)^- net ionic charge | +1 | -3 | -1 alternate names | ammonium | ammonium(1+) | protonated ammonia | orthophosphate | tetraoxophosphate | tetraoxidophosphate | phosphate | phosphate(3-) | trioxonitrate | trioxidonitrate | nitrate | nitrate(1-)
Ionic radii

| ammonium cation | phosphate anion | nitrate anion thermochemical radius | 137 pm | 238 pm | 179 pm
Units

Other properties
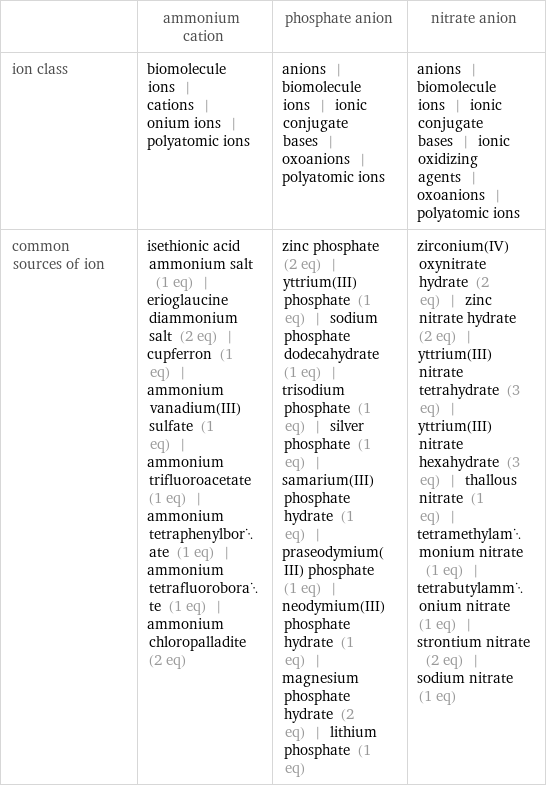
| ammonium cation | phosphate anion | nitrate anion ion class | biomolecule ions | cations | onium ions | polyatomic ions | anions | biomolecule ions | ionic conjugate bases | oxoanions | polyatomic ions | anions | biomolecule ions | ionic conjugate bases | ionic oxidizing agents | oxoanions | polyatomic ions common sources of ion | isethionic acid ammonium salt (1 eq) | erioglaucine diammonium salt (2 eq) | cupferron (1 eq) | ammonium vanadium(III) sulfate (1 eq) | ammonium trifluoroacetate (1 eq) | ammonium tetraphenylborate (1 eq) | ammonium tetrafluoroborate (1 eq) | ammonium chloropalladite (2 eq) | zinc phosphate (2 eq) | yttrium(III) phosphate (1 eq) | sodium phosphate dodecahydrate (1 eq) | trisodium phosphate (1 eq) | silver phosphate (1 eq) | samarium(III) phosphate hydrate (1 eq) | praseodymium(III) phosphate (1 eq) | neodymium(III) phosphate hydrate (1 eq) | magnesium phosphate hydrate (2 eq) | lithium phosphate (1 eq) | zirconium(IV) oxynitrate hydrate (2 eq) | zinc nitrate hydrate (2 eq) | yttrium(III)nitrate tetrahydrate (3 eq) | yttrium(III) nitrate hexahydrate (3 eq) | thallous nitrate (1 eq) | tetramethylammonium nitrate (1 eq) | tetrabutylammonium nitrate (1 eq) | strontium nitrate (2 eq) | sodium nitrate (1 eq)