Input interpretation
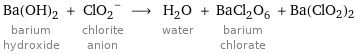
Ba(OH)_2 barium hydroxide + (ClO_2)^- chlorite anion ⟶ H_2O water + BaCl_2O_6 barium chlorate + Ba(ClO2)2
Chemical names and formulas
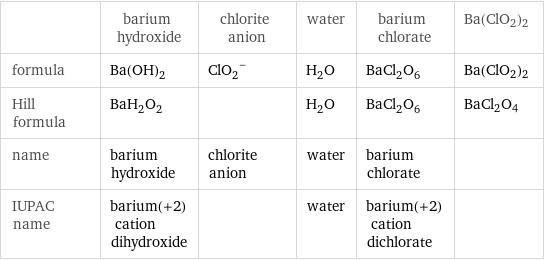
| barium hydroxide | chlorite anion | water | barium chlorate | Ba(ClO2)2 formula | Ba(OH)_2 | (ClO_2)^- | H_2O | BaCl_2O_6 | Ba(ClO2)2 Hill formula | BaH_2O_2 | | H_2O | BaCl_2O_6 | BaCl2O4 name | barium hydroxide | chlorite anion | water | barium chlorate | IUPAC name | barium(+2) cation dihydroxide | | water | barium(+2) cation dichlorate |