Input interpretation

top 10 chemicals | by NFPA reactivity rating
Result
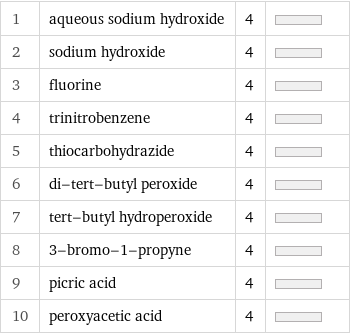
1 | aqueous sodium hydroxide | 4 | 2 | sodium hydroxide | 4 | 3 | fluorine | 4 | 4 | trinitrobenzene | 4 | 5 | thiocarbohydrazide | 4 | 6 | di-tert-butyl peroxide | 4 | 7 | tert-butyl hydroperoxide | 4 | 8 | 3-bromo-1-propyne | 4 | 9 | picric acid | 4 | 10 | peroxyacetic acid | 4 |
Structure diagrams
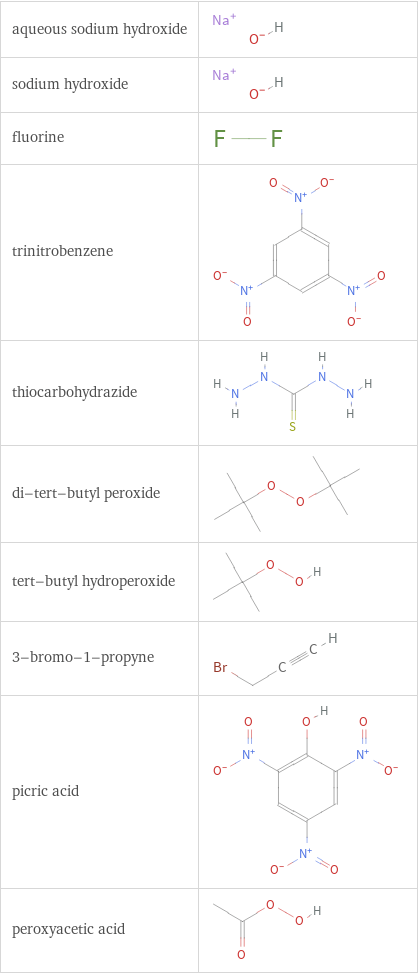
Structure diagrams
Basic properties
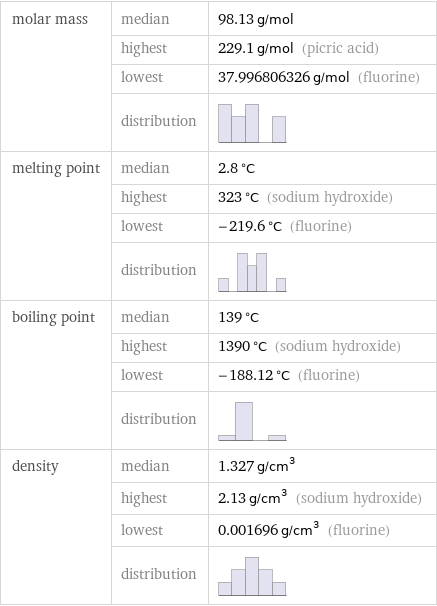
molar mass | median | 98.13 g/mol | highest | 229.1 g/mol (picric acid) | lowest | 37.996806326 g/mol (fluorine) | distribution | melting point | median | 2.8 °C | highest | 323 °C (sodium hydroxide) | lowest | -219.6 °C (fluorine) | distribution | boiling point | median | 139 °C | highest | 1390 °C (sodium hydroxide) | lowest | -188.12 °C (fluorine) | distribution | density | median | 1.327 g/cm^3 | highest | 2.13 g/cm^3 (sodium hydroxide) | lowest | 0.001696 g/cm^3 (fluorine) | distribution |
Units

pH sensitivity

| pH range | color change trinitrobenzene | 12 to 14 | colorless to orange