Input interpretation

refrigerants | vapor pressure
Summary
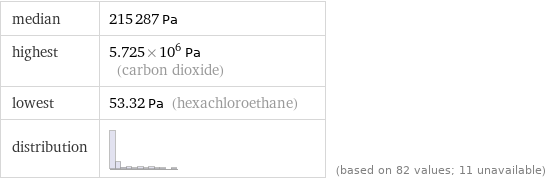
median | 215287 Pa highest | 5.725×10^6 Pa (carbon dioxide) lowest | 53.32 Pa (hexachloroethane) distribution | | (based on 82 values; 11 unavailable)
Entities with missing values

hydrogen | neon | nitrogen | vinyl chloride | 1, 2-difluoroethane | krypton | tetrafluoromethane | 1, 1-dichloroethane | 2, 2, 2-trifluoroethyl methyl ether | dichlorodifluoromethane | hexafluoropropene, trimer (total: 11)
Distribution plots
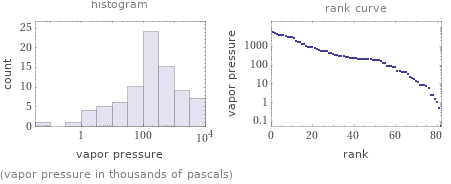
(vapor pressure in thousands of pascals)
Vapor pressure rankings
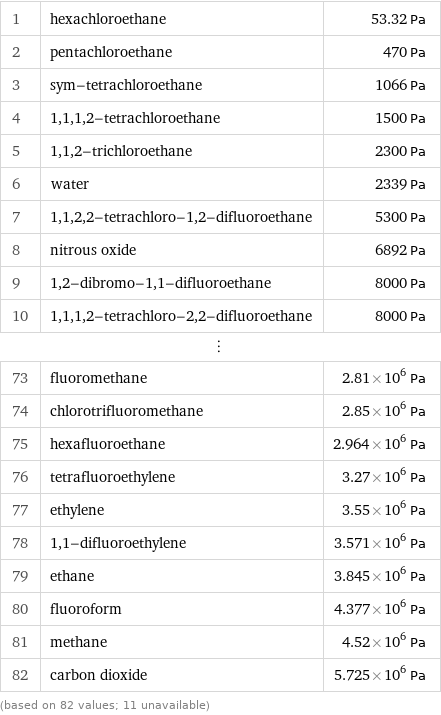
1 | hexachloroethane | 53.32 Pa 2 | pentachloroethane | 470 Pa 3 | sym-tetrachloroethane | 1066 Pa 4 | 1, 1, 1, 2-tetrachloroethane | 1500 Pa 5 | 1, 1, 2-trichloroethane | 2300 Pa 6 | water | 2339 Pa 7 | 1, 1, 2, 2-tetrachloro-1, 2-difluoroethane | 5300 Pa 8 | nitrous oxide | 6892 Pa 9 | 1, 2-dibromo-1, 1-difluoroethane | 8000 Pa 10 | 1, 1, 1, 2-tetrachloro-2, 2-difluoroethane | 8000 Pa ⋮ | | 73 | fluoromethane | 2.81×10^6 Pa 74 | chlorotrifluoromethane | 2.85×10^6 Pa 75 | hexafluoroethane | 2.964×10^6 Pa 76 | tetrafluoroethylene | 3.27×10^6 Pa 77 | ethylene | 3.55×10^6 Pa 78 | 1, 1-difluoroethylene | 3.571×10^6 Pa 79 | ethane | 3.845×10^6 Pa 80 | fluoroform | 4.377×10^6 Pa 81 | methane | 4.52×10^6 Pa 82 | carbon dioxide | 5.725×10^6 Pa (based on 82 values; 11 unavailable)
Unit conversions for median vapor pressure 215287 Pa
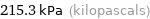
215.3 kPa (kilopascals)
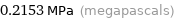
0.2153 MPa (megapascals)

215287 N/m^2 (newtons per square meter)
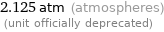
2.125 atm (atmospheres) (unit officially deprecated)

2.195 at (technical atmospheres) (unit officially deprecated)
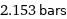
2.153 bars
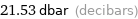
21.53 dbar (decibars)
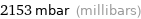
2153 mbar (millibars)
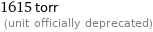
1615 torr (unit officially deprecated)
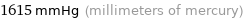
1615 mmHg (millimeters of mercury)
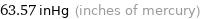
63.57 inHg (inches of mercury)
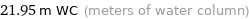
21.95 m WC (meters of water column)

0.02195 km WC (kilometers of water column)

864.3 in WC (inches of water column (conventional))
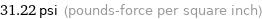
31.22 psi (pounds-force per square inch)
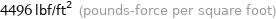
4496 lbf/ft^2 (pounds-force per square foot)
Comparisons for median vapor pressure 215287 Pa

≈ (0.6 to 1) × pressure inside a typical soda can at room temperature ( 207 to 380 kPa )
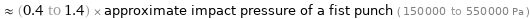
≈ (0.4 to 1.4) × approximate impact pressure of a fist punch ( 150000 to 550000 Pa )
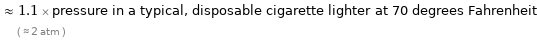
≈ 1.1 × pressure in a typical, disposable cigarette lighter at 70 degrees Fahrenheit ( ≈ 2 atm )
Corresponding quantity
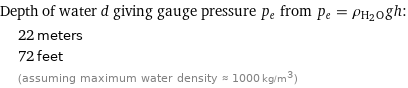
Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 22 meters | 72 feet | (assuming maximum water density ≈ 1000 kg/m^3)