Input interpretation
magnesium hydroxide | nitric acid
Chemical names and formulas
![| magnesium hydroxide | nitric acid formula | Mg(OH)_2 | HNO_3 Hill formula | H_2MgO_2 | HNO_3 name | magnesium hydroxide | nitric acid IUPAC name | magnesium dihydroxide | nitric acid alternate names | magnesia magma | magnesia, [Milk of] | magnesium dihydroxide | magnesium hydrate | milk of magnesia | anhydrous nitric acid | azotic acid | hydrogen nitrate | nital | spirit of nitre mass fractions | H (hydrogen) 3.46% | Mg (magnesium) 41.7% | O (oxygen) 54.9% | H (hydrogen) 1.6% | N (nitrogen) 22.2% | O (oxygen) 76.2%](../image_source/fe86c5b7c1f05eeadb331df32e8d7ef3.png)
| magnesium hydroxide | nitric acid formula | Mg(OH)_2 | HNO_3 Hill formula | H_2MgO_2 | HNO_3 name | magnesium hydroxide | nitric acid IUPAC name | magnesium dihydroxide | nitric acid alternate names | magnesia magma | magnesia, [Milk of] | magnesium dihydroxide | magnesium hydrate | milk of magnesia | anhydrous nitric acid | azotic acid | hydrogen nitrate | nital | spirit of nitre mass fractions | H (hydrogen) 3.46% | Mg (magnesium) 41.7% | O (oxygen) 54.9% | H (hydrogen) 1.6% | N (nitrogen) 22.2% | O (oxygen) 76.2%
Structure diagrams
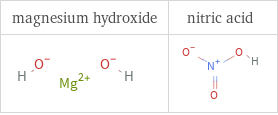
Structure diagrams
3D structure
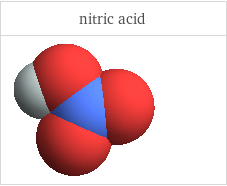
3D structure
Basic properties
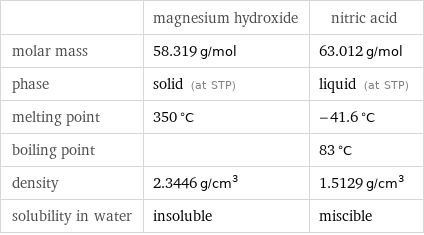
| magnesium hydroxide | nitric acid molar mass | 58.319 g/mol | 63.012 g/mol phase | solid (at STP) | liquid (at STP) melting point | 350 °C | -41.6 °C boiling point | | 83 °C density | 2.3446 g/cm^3 | 1.5129 g/cm^3 solubility in water | insoluble | miscible
Units

Liquid properties
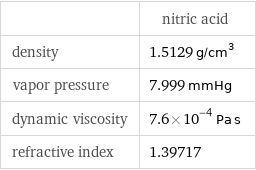
| nitric acid density | 1.5129 g/cm^3 vapor pressure | 7.999 mmHg dynamic viscosity | 7.6×10^-4 Pa s refractive index | 1.39717
Units
Thermodynamic properties
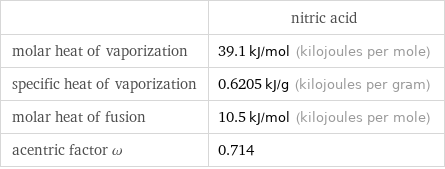
| nitric acid molar heat of vaporization | 39.1 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.6205 kJ/g (kilojoules per gram) molar heat of fusion | 10.5 kJ/mol (kilojoules per mole) acentric factor ω | 0.714
Solid properties
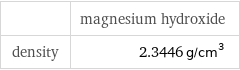
| magnesium hydroxide density | 2.3446 g/cm^3
Units

Chemical identifiers
![| magnesium hydroxide | nitric acid CAS number | 1309-42-8 | 7697-37-2 PubChem CID number | 14791 | 944 PubChem SID number | 24882320 | 24853516 SMILES identifier | [OH-].[OH-].[Mg+2] | [N+](=O)(O)[O-]](../image_source/9f8d6056cb7a3507a855df9975487349.png)
| magnesium hydroxide | nitric acid CAS number | 1309-42-8 | 7697-37-2 PubChem CID number | 14791 | 944 PubChem SID number | 24882320 | 24853516 SMILES identifier | [OH-].[OH-].[Mg+2] | [N+](=O)(O)[O-]
NFPA label

NFPA label
Toxicity properties

| nitric acid short-term exposure limit | 10 mg/m^3 threshold limit value | 2 ppmv
Units