Input interpretation

tribromide anion | structure diagram
Result
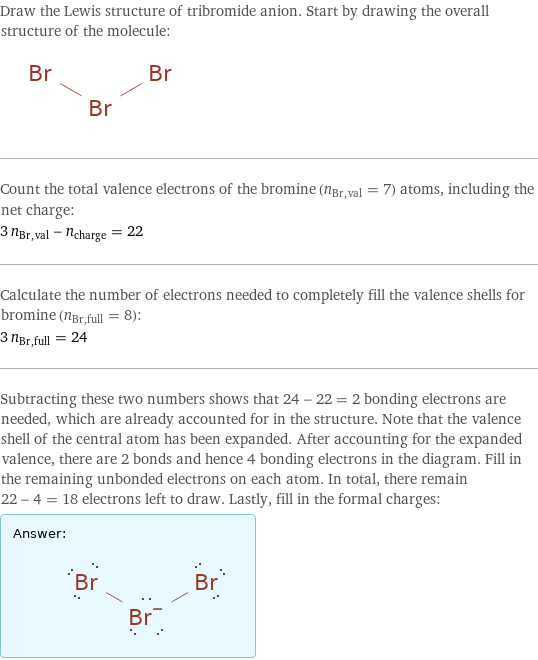
Draw the Lewis structure of tribromide anion. Start by drawing the overall structure of the molecule: Count the total valence electrons of the bromine (n_Br, val = 7) atoms, including the net charge: 3 n_Br, val - n_charge = 22 Calculate the number of electrons needed to completely fill the valence shells for bromine (n_Br, full = 8): 3 n_Br, full = 24 Subtracting these two numbers shows that 24 - 22 = 2 bonding electrons are needed, which are already accounted for in the structure. Note that the valence shell of the central atom has been expanded. After accounting for the expanded valence, there are 2 bonds and hence 4 bonding electrons in the diagram. Fill in the remaining unbonded electrons on each atom. In total, there remain 22 - 4 = 18 electrons left to draw. Lastly, fill in the formal charges: Answer: | |