Input interpretation
triethylaluminum
Chemical names and formulas
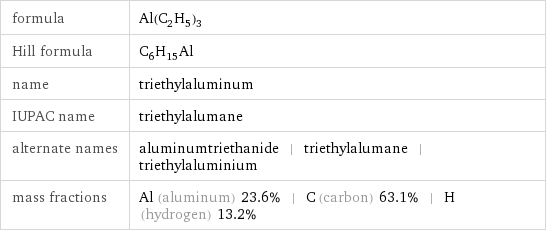
formula | Al(C_2H_5)_3 Hill formula | C_6H_15Al name | triethylaluminum IUPAC name | triethylalumane alternate names | aluminumtriethanide | triethylalumane | triethylaluminium mass fractions | Al (aluminum) 23.6% | C (carbon) 63.1% | H (hydrogen) 13.2%
Lewis structure
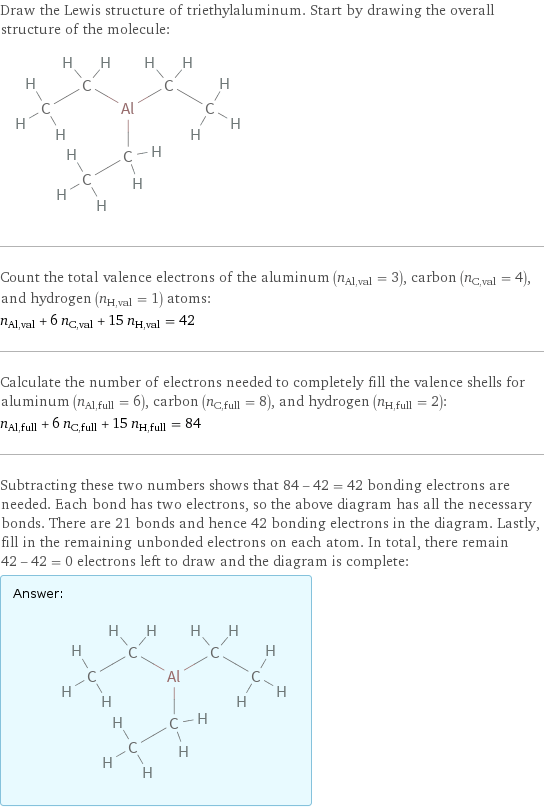
Draw the Lewis structure of triethylaluminum. Start by drawing the overall structure of the molecule: Count the total valence electrons of the aluminum (n_Al, val = 3), carbon (n_C, val = 4), and hydrogen (n_H, val = 1) atoms: n_Al, val + 6 n_C, val + 15 n_H, val = 42 Calculate the number of electrons needed to completely fill the valence shells for aluminum (n_Al, full = 6), carbon (n_C, full = 8), and hydrogen (n_H, full = 2): n_Al, full + 6 n_C, full + 15 n_H, full = 84 Subtracting these two numbers shows that 84 - 42 = 42 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 21 bonds and hence 42 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 42 - 42 = 0 electrons left to draw and the diagram is complete: Answer: | |
Basic properties
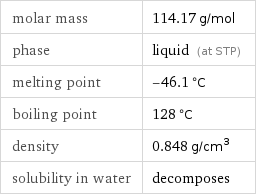
molar mass | 114.17 g/mol phase | liquid (at STP) melting point | -46.1 °C boiling point | 128 °C density | 0.848 g/cm^3 solubility in water | decomposes
Units

Liquid properties (at STP)
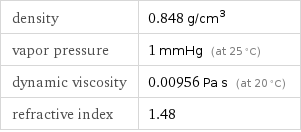
density | 0.848 g/cm^3 vapor pressure | 1 mmHg (at 25 °C) dynamic viscosity | 0.00956 Pa s (at 20 °C) refractive index | 1.48
Units

Thermodynamic properties
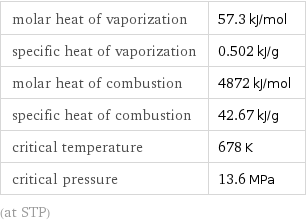
molar heat of vaporization | 57.3 kJ/mol specific heat of vaporization | 0.502 kJ/g molar heat of combustion | 4872 kJ/mol specific heat of combustion | 42.67 kJ/g critical temperature | 678 K critical pressure | 13.6 MPa (at STP)
Chemical identifiers
CC InChI identifier | InChI=1/3C2H5.Al/c3*1-2;/h3*1H2, 2H3;/rC6H15Al/c1-4-7(5-2)6-3/h4-6H2, 1-3H3 MDL number | MFCD00009015](../image_source/b8c215dcd3ce33b2a73592a3d1942df7.png)
CAS number | 97-93-8 Beilstein number | 3587229 PubChem CID number | 16682930 PubChem SID number | 24851559 SMILES identifier | CC[Al](CC)CC InChI identifier | InChI=1/3C2H5.Al/c3*1-2;/h3*1H2, 2H3;/rC6H15Al/c1-4-7(5-2)6-3/h4-6H2, 1-3H3 MDL number | MFCD00009015
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 4 NFPA reactivity rating | 3 NFPA hazards | water reactive
Safety properties

flash point | -18.33 °C
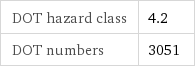
DOT hazard class | 4.2 DOT numbers | 3051
Toxicity properties

RTECS classes | other