Input interpretation
ammonium sulfamate
Chemical names and formulas
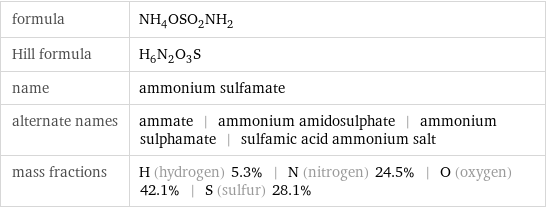
formula | NH_4OSO_2NH_2 Hill formula | H_6N_2O_3S name | ammonium sulfamate alternate names | ammate | ammonium amidosulphate | ammonium sulphamate | sulfamic acid ammonium salt mass fractions | H (hydrogen) 5.3% | N (nitrogen) 24.5% | O (oxygen) 42.1% | S (sulfur) 28.1%
Structure diagram
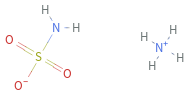
Structure diagram
Basic properties
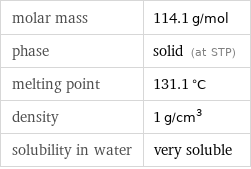
molar mass | 114.1 g/mol phase | solid (at STP) melting point | 131.1 °C density | 1 g/cm^3 solubility in water | very soluble
Units

Solid properties (at STP)

density | 1 g/cm^3
Units

Thermodynamic properties

molar heat of fusion | 15.2 kJ/mol specific heat of fusion | 0.1332 kJ/g (at STP)
Chemical identifiers
![CAS number | 7773-06-0 PubChem CID number | 24482 SMILES identifier | [NH4+].NS(=O)(=O)[O-] InChI identifier | InChI=1/H3NO3S.H3N/c1-5(2, 3)4;/h(H3, 1, 2, 3, 4);1H3/fH2NO3S.H4N/h1H2;1H/q-1;+1 EU number | 231-871-7 Gmelin number | 180976 RTECS number | WO6125000](../image_source/a2946c7b5d72dfa255c5c2dce420b83b.png)
CAS number | 7773-06-0 PubChem CID number | 24482 SMILES identifier | [NH4+].NS(=O)(=O)[O-] InChI identifier | InChI=1/H3NO3S.H3N/c1-5(2, 3)4;/h(H3, 1, 2, 3, 4);1H3/fH2NO3S.H4N/h1H2;1H/q-1;+1 EU number | 231-871-7 Gmelin number | 180976 RTECS number | WO6125000
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 1 NFPA reactivity rating | 0
Toxicity properties
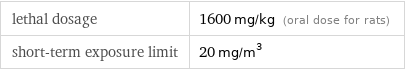
lethal dosage | 1600 mg/kg (oral dose for rats) short-term exposure limit | 20 mg/m^3

probable lethal dose for man | 600 mL (milliliters) long-term exposure limit | 10 mg/m^3 (over 8 hours) RTECS classes | agricultural chemical and pesticide