Input interpretation
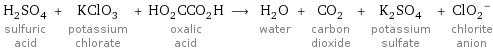
H_2SO_4 sulfuric acid + KClO_3 potassium chlorate + HO_2CCO_2H oxalic acid ⟶ H_2O water + CO_2 carbon dioxide + K_2SO_4 potassium sulfate + (ClO_2)^- chlorite anion
Chemical names and formulas
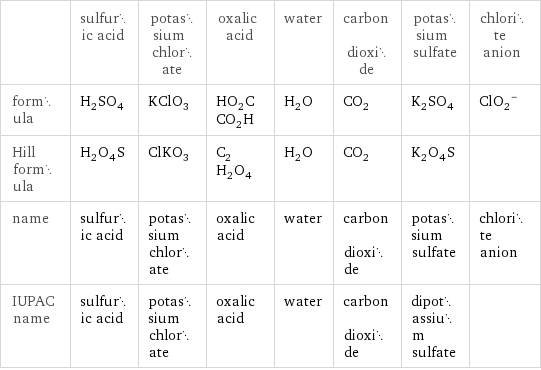
| sulfuric acid | potassium chlorate | oxalic acid | water | carbon dioxide | potassium sulfate | chlorite anion formula | H_2SO_4 | KClO_3 | HO_2CCO_2H | H_2O | CO_2 | K_2SO_4 | (ClO_2)^- Hill formula | H_2O_4S | ClKO_3 | C_2H_2O_4 | H_2O | CO_2 | K_2O_4S | name | sulfuric acid | potassium chlorate | oxalic acid | water | carbon dioxide | potassium sulfate | chlorite anion IUPAC name | sulfuric acid | potassium chlorate | oxalic acid | water | carbon dioxide | dipotassium sulfate |