Input interpretation
cyhalothrin
Chemical names and formulas
![formula | C_23H_19ClF_3NO_3 name | cyhalothrin IUPAC name | [cyano-(3-phenoxyphenyl)methyl] 3-[(Z)-2-chloro-3, 3, 3-trifluoro-prop-1-enyl]-2, 2-dimethyl-cyclopropane-1-carboxylate mass fractions | C (carbon) 61.4% | Cl (chlorine) 7.88% | F (fluorine) 12.7% | H (hydrogen) 4.26% | N (nitrogen) 3.11% | O (oxygen) 10.7%](../image_source/3fbfdbf0708fc92fbaae3ce27bb50c64.png)
formula | C_23H_19ClF_3NO_3 name | cyhalothrin IUPAC name | [cyano-(3-phenoxyphenyl)methyl] 3-[(Z)-2-chloro-3, 3, 3-trifluoro-prop-1-enyl]-2, 2-dimethyl-cyclopropane-1-carboxylate mass fractions | C (carbon) 61.4% | Cl (chlorine) 7.88% | F (fluorine) 12.7% | H (hydrogen) 4.26% | N (nitrogen) 3.11% | O (oxygen) 10.7%
Lewis structure
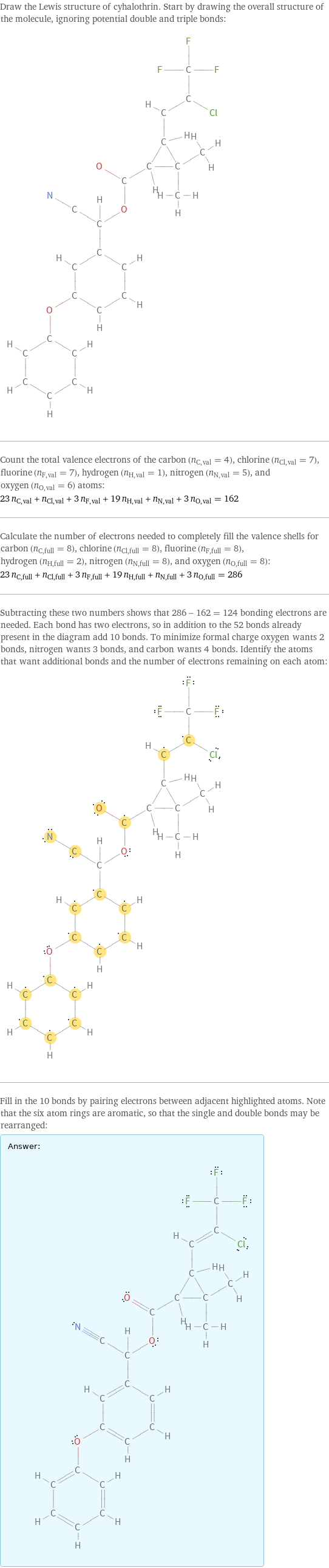
Draw the Lewis structure of cyhalothrin. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 23 n_C, val + n_Cl, val + 3 n_F, val + 19 n_H, val + n_N, val + 3 n_O, val = 162 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 23 n_C, full + n_Cl, full + 3 n_F, full + 19 n_H, full + n_N, full + 3 n_O, full = 286 Subtracting these two numbers shows that 286 - 162 = 124 bonding electrons are needed. Each bond has two electrons, so in addition to the 52 bonds already present in the diagram add 10 bonds. To minimize formal charge oxygen wants 2 bonds, nitrogen wants 3 bonds, and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 10 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom rings are aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
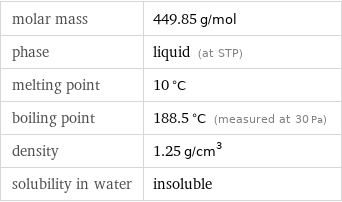
molar mass | 449.85 g/mol phase | liquid (at STP) melting point | 10 °C boiling point | 188.5 °C (measured at 30 Pa) density | 1.25 g/cm^3 solubility in water | insoluble
Units

Liquid properties (at STP)

density | 1.25 g/cm^3 vapor pressure | 8×10^-9 mmHg (at 25 °C)
Units

Chemical identifiers
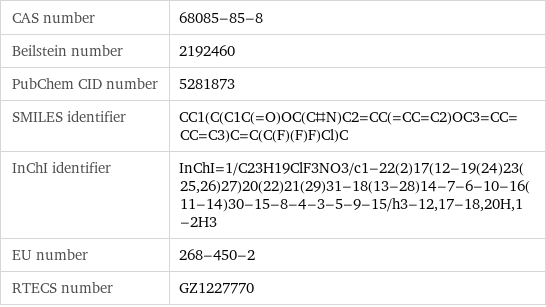
CAS number | 68085-85-8 Beilstein number | 2192460 PubChem CID number | 5281873 SMILES identifier | CC1(C(C1C(=O)OC(C#N)C2=CC(=CC=C2)OC3=CC=CC=C3)C=C(C(F)(F)F)Cl)C InChI identifier | InChI=1/C23H19ClF3NO3/c1-22(2)17(12-19(24)23(25, 26)27)20(22)21(29)31-18(13-28)14-7-6-10-16(11-14)30-15-8-4-3-5-9-15/h3-12, 17-18, 20H, 1-2H3 EU number | 268-450-2 RTECS number | GZ1227770
Safety properties

flash point | 80 °C

DOT hazard class | 6.1 DOT numbers | 2902
Toxicity properties

RTECS classes | agricultural chemical and pesticide