Input interpretation
calcium iodide
Chemical names and formulas

formula | CaI_2 name | calcium iodide IUPAC name | calcium diiodide alternate names | calcium diiodide | calcium iodideanhydrous | yokaron mass fractions | Ca (calcium) 13.6% | I (iodine) 86.4%
Structure diagram
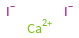
Structure diagram
Basic properties
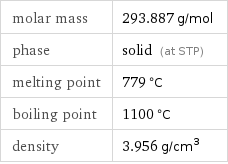
molar mass | 293.887 g/mol phase | solid (at STP) melting point | 779 °C boiling point | 1100 °C density | 3.956 g/cm^3
Units

Solid properties (at STP)

density | 3.956 g/cm^3
Units

Thermodynamic properties
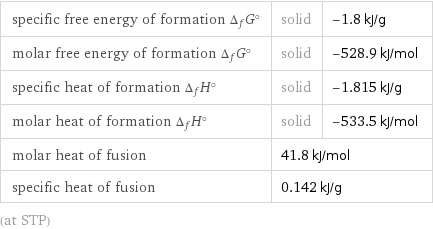
specific free energy of formation Δ_fG° | solid | -1.8 kJ/g molar free energy of formation Δ_fG° | solid | -528.9 kJ/mol specific heat of formation Δ_fH° | solid | -1.815 kJ/g molar heat of formation Δ_fH° | solid | -533.5 kJ/mol molar heat of fusion | 41.8 kJ/mol | specific heat of fusion | 0.142 kJ/g | (at STP)
Chemical identifiers
![CAS number | 10102-68-8 PubChem CID number | 66244 PubChem SID number | 24867542 SMILES identifier | [Ca+2].[I-].[I-] InChI identifier | InChI=1/Ca.2HI/h;2*1H/q+2;;/p-2/fCa.2I/h;2*1h/qm;2*-1 MDL number | MFCD00010910](../image_source/6b77559d8e6fda8a2904a8660cf1ff62.png)
CAS number | 10102-68-8 PubChem CID number | 66244 PubChem SID number | 24867542 SMILES identifier | [Ca+2].[I-].[I-] InChI identifier | InChI=1/Ca.2HI/h;2*1H/q+2;;/p-2/fCa.2I/h;2*1h/qm;2*-1 MDL number | MFCD00010910
NFPA label

NFPA label
Ion equivalents

Ca^(2+) (calcium cation) | 1 I^- (iodide anion) | 2